President’s Cancer Panel Addresses Gaps in Cancer Screening for All Americans
, by Jennifer K. Loukissas, M.P.P.
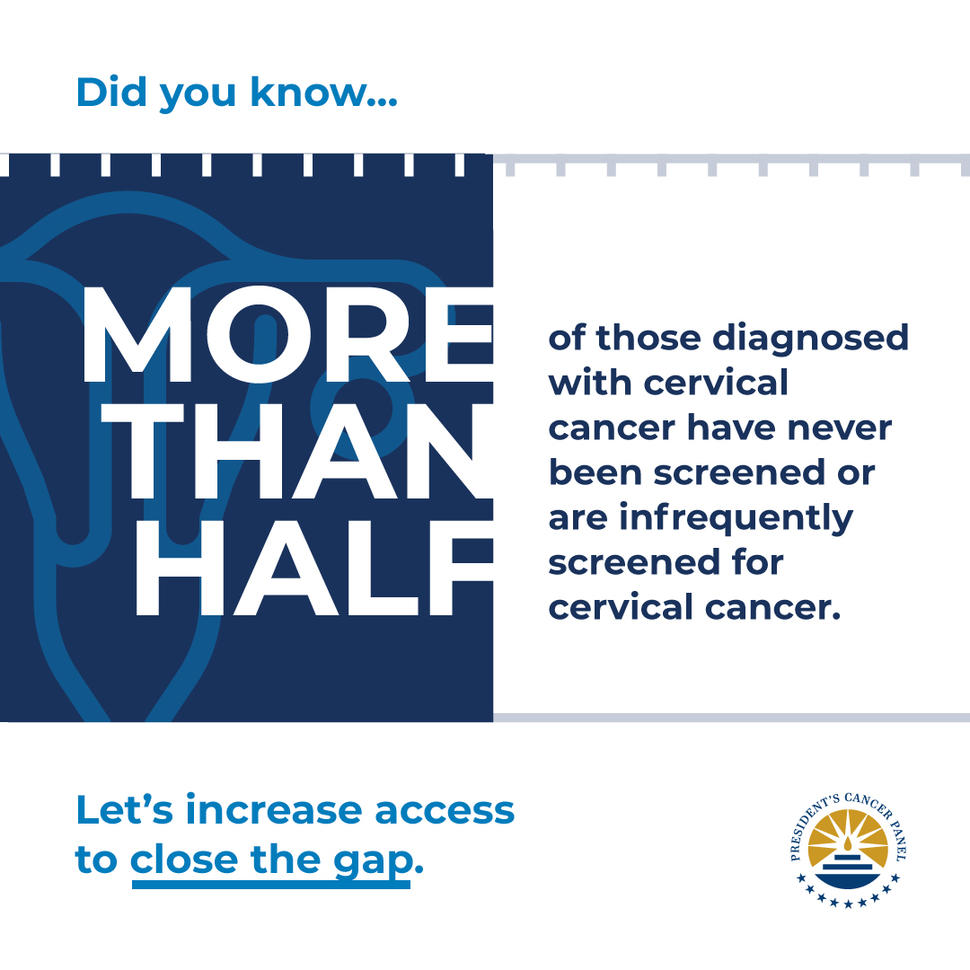
In February 2022, the President’s Cancer Panel released their report “Closing Gaps in Cancer Screening: Connecting People, Communities, and Systems to Improve Equity and Access,” outlining opportunities to improve the resilience and equity of screenings for cancers. The National Cancer Act of 1971 established the Panel to monitor the activities of the National Cancer Program and report to the President of the United States on barriers to progress in reducing the burden of cancer.
The report identifies four opportunities to improve the screening process, the result of a two-year fact-finding Meeting Series, “Improving Resilience and Equity in Cancer Screenings,” to address the suboptimal implementation of cancer screening—evident prior to and significantly amplified by the COVID-19 pandemic.
The goals are to:
- Improve and align cancer screening communication.
- Facilitate equitable access to cancer screening.
- Strengthen workforce collaborations to support cancer screening and risk assessment.
- Create health information technology that promotes appropriate cancer risk assessment and screening.
Panel Chair Dr. John P. Williams describes the Panel’s recommendations in the Cancer Currents blog.
Nicolas Wentzensen, M.D., Ph.D., Head of the Clinical Epidemiology Unit and senior investigator and Deputy Chief in the Clinical Genetics Branch, co-chaired the Cervical Cancer Planning Subgroup, which developed recommendations related to cervical cancer screening and management throughout 2020 and held a public meeting in November 2020. Dr. Wentzensen, an expert in gynecologic cancers, investigates natural history and multi-step carcinogenesis, novel biomarkers for precancer and cancer, and develops risk estimates underlying cancer screening and management. He conducts research related to improving screening, including the Cancer Moonshot project to Accelerate Cervical Cancer Control, and leads the Enduring Guidelines effort focused on advancing screening and clinical management guidelines. He is also highly engaged in the development of the WHO cervical cancer screening and management guidelines.
Working Group and Federal Collaborative
Several activities within the cervical cancer prevention landscape are underway to address the first three goals identified by the Panel. The American Cancer Society (ACS) convened the Cervical Cancer Screening Initiative (CCSI)—a two-year project involving advocates, clinicians, researchers, and other experts to speed the transition to primary HPV screening in the United States. Several experts in DCEG participate across the six working groups: Megan Clarke, Ph.D., Earl Stadtman tenure-track investigator in CEU/CGB, serves on the Moving from Cytology Alone workgroup and Jennifer K. Loukissas, M.P.P., Communications Chief, is a member of the patient engagement working group. Dr. Wentzensen serves on the steering committee.
Concurrently, the Federal Cervical Cancer Collaborative, a cross-agency collaboration led by the Health Resources and Services Administration (HRSA), seeks to strengthen cervical cancer prevention, screening, and management in safety-net settings of care. From DCEG, Ms. Loukissas and Dr. Wentzensen are joined by Justine E. Yu, Ph.D., and Elise Tookmanian, Ph.D., both of the communications team, and Christine Cochrane, M.S., program analyst in CEU/CGB. Representatives from the Division of Cancer Control and Population Sciences include Veronica Chollette, R.N., M.S. and Sarah Kobrin, Ph.D., M.P.H. The full complement of partners include:
- HRSA Office of Women’s Health
- National Institutes of Health (NIH) National Cancer Institute
- NIH Office for Research on Women’s Health
- Department of Health and Human Services (HHS) Office of Population Affairs in the Office of the Assistant Secretary for Health
- HRSA Office of Regional Operations
- Centers for Disease Control and Prevention (CDC) Division of Cancer Prevention and Control
The Collaborative will hold a series of roundtables with stakeholders and organizations composed of clinicians, public health personnel, health center directors, and researchers, through the spring of 2022 to inform the development of a federal opportunities report and provider-facing toolkit for safety-net settings of care. In year-two, the Collaborative will generate a patient-facing toolkit.