What are the Enduring Guidelines?
The Enduring Consensus Cervical Cancer Screening and Management Guidelines (known as the Enduring Guidelines) are a collection of updates to the 2019 ASCCP Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. The Enduring Guidelines are generated by a consensus committee of experts tasked with performing, evaluating, and ratifying risk estimations for cervical cancer screening tests; their decisions on new tests and associated risk estimates will be posted to this web page, along with links to the supporting scientific literature.
The Enduring Guidelines are designed to recognize and reduce disparities in cervical cancer prevention.
Why are Enduring Guidelines Necessary?
Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors are predicated upon the principle of ‘equal management for equal risk:’ clinical management is based on estimated risk within a specific time interval, rather than the results of a specific screening or clinical test.
In the 2019 ASCCP Risk-Based Management Consensus Guidelines, the process to estimate risk was explicitly separated from the establishment of clinical action thresholds to allow more frequent updates to the guidelines. The risk estimates that trigger management actions, called Clinical Action Thresholds, were defined by a consensus group including stakeholders from 20 professional societies, federal organizations, and patient advocacy groups.
The full risk tables are available online and linked below.
The Clinical Action Thresholds are expected to be in place for the next decade (See Figure below). Risk estimations for a limited set of tests (mainly HPV and cytology) and other variables were generated by investigators at the National Cancer Institute (NCI). These estimates, in the context of the Clinical Action Thresholds, underlie the current set of guidelines.
As new technologies become available and population characteristics change, e.g. increasing impact of HPV vaccination, new risk estimates will be needed frequently, and existing guidelines updated. The Enduring Guidelines Effort addresses this ongoing need.
Publications from the Enduring Guidelines Effort
Approach and Methods
Wentzensen N et al. Enduring Consensus Guidelines for Cervical Cancer Screening and Management: Introduction to the Scope and Process. J Low Genit Tract Dis. 2024.
Egemen D et al. Risk-Based Cervical Consensus Guidelines: Methods to Determine Management if Less Than 5 Years of Data Are Available. J Low Genit Tract Dis. 2022.
Enduring Guidelines Recommendations
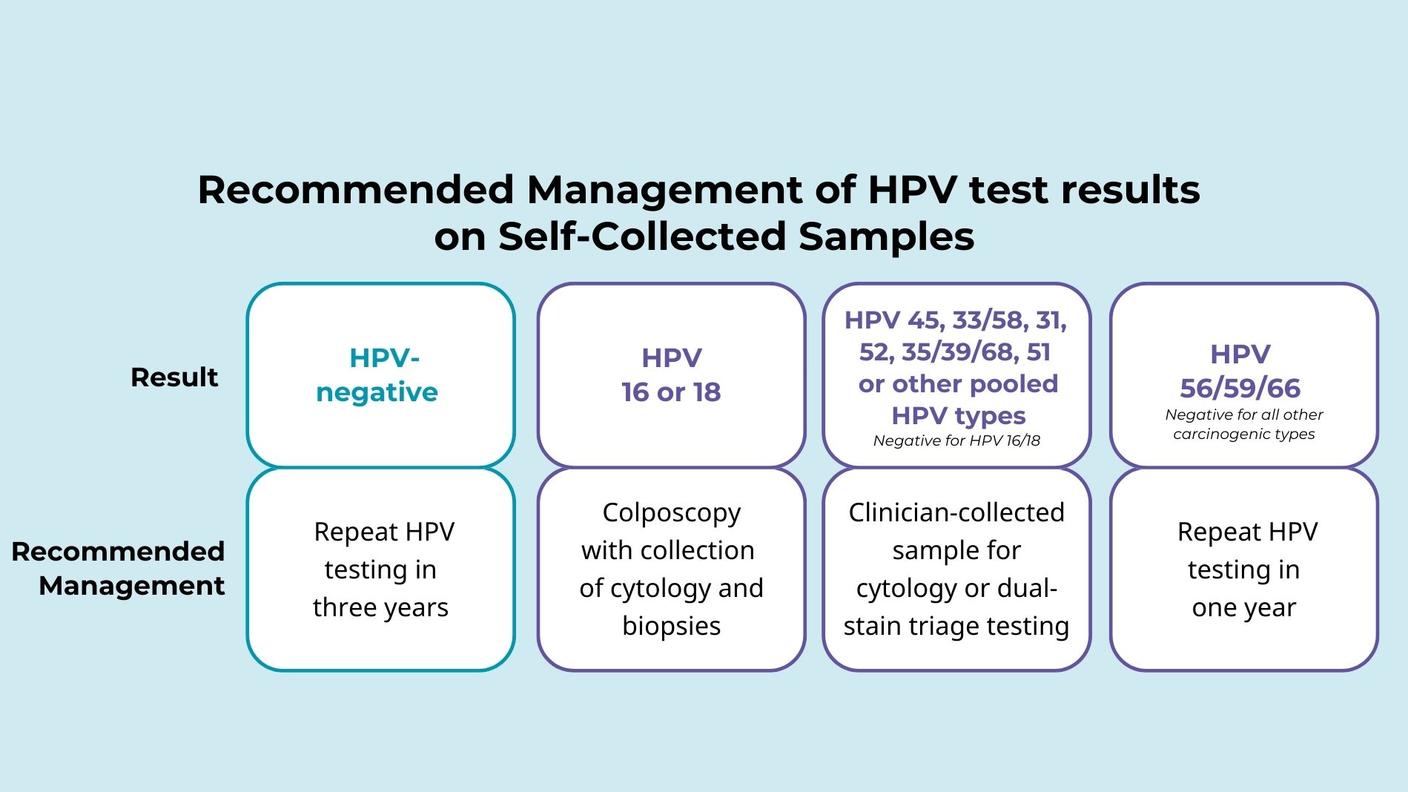
- Self-collection: Wentzensen N et al. Self-Collected Vaginal Specimens for HPV Testing: Recommendations From the Enduring Consensus Cervical Cancer Screening and Management Guidelines Committee. J Low Genit Tract Dis. 2025.
- Extended Genotyping: Massad LS et al. Applying Results of Extended Genotyping to Management of Positive Cervicovaginal Human Papillomavirus Test Results: Enduring Guidelines. J Low Genit Tract Dis. 2025.
- Dual Stain: Clarke M et al. Recommendations for Use of p16/Ki67 Dual Stain for Management of Individuals Testing Positive for Human Papillomavirus. J Low Genit Tract Dis. 2024.
Participating Organizations
- American Academy of Family Physicians
- American Cancer Society
- American College of Nurse-Midwives
- American College of Obstetricians and Gynecologists
- American Sexual Health Association
- American Society for Clinical Pathology
- American Society of Cytopathology
- ASCCP
- Association for Physician Assistants in Obstetrics and Gynecology (APAOG)
- Centers for Disease Control & Prevention
- Cervivor
- College of American Pathologists
- National Cancer Institute
- Nurse Practitioners in Women's Health
- Nurses for Sexual and Reproductive Health
- Papanicolaou Society of Cytopathology
- Planned Parenthood Federation of America
- Society of Gynecologic Oncology
- Team Maureen
- Women Veterans Health Strategic Healthcare Group
Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines
The 2019 ASCCP Risk-Based Management Consensus Guidelines (Perkins and Guido et al.) for the management of cervical cancer screening abnormalities recommend 1 of 6 clinical actions (treatment, optional treatment or colposcopy/biopsy, colposcopy/biopsy, 1-year surveillance, 3-year surveillance, 5-year return to regular screening) based on the risk of cervical intraepithelial neoplasia grade 3, adenocarcinoma in situ, or cancer (CIN 3+) for the many different combinations of current and recent past screening results.
The tables display risk estimates for CIN 3+, CIN 2+, and cancer, for every possible combination of test results, as the data permits. These risk scores are obtained at time points, 0 (immediate), 1, 2, 3, 4, and 5 years. Each risk estimate is presented with its corresponding standard error (SE) and 95% lower (LL95) and upper (UL95) confidence interval. Test results are ordered chronologically; therefore, in each table, the leftmost column presents the oldest test result in the screening history of the patient while the rightmost column(s) (among the test results) displays the current test result. The total sample size (N) in each category and each screening result as a percentage (%) of total screened, the total number of patients informative in risk estimation (N Informative) are listed in the columns following the test results. Total number of observed CIN 2+, CIN 3+, and cancer cases are displayed together with the breakdown of prevalence, incidence, and unknown prevalence/incidence in the subsequent columns. Following the risk estimates columns, recommended management and “Recommendation Confidence Score” (for more details about this score please refer to Egemen et al.) are listed. Sampling weights are used for the sample from which we obtained the risk estimates for the HPV genotyping test results as explained in Demarco et al. The raw sample sizes (without the sampling weights) are presented at the rightmost columns of each table. The statistical methods for risk estimation are explained in Cheung et al.
Main Tables
The 2019 ASCCP Risk-Based Management Consensus Guidelines for the management of cervical cancer screening abnormalities recommend one of six clinical actions based on the risk of cervical intraepithelial neoplasia grade 3 (CIN 3), adenocarcinoma in situ, or cancer (CIN 3+) for the many different combinations of current and recent past screening results. Those clinical actions are:
- Treatment
- Optional treatment or colposcopy/biopsy
- Colposcopy/biopsy
- 1-year surveillance
- 3-year surveillance
- 5-year return to regular screening
The tables linked below display the risk estimates of CIN 3+, as well as CIN 2+ and cancer, for every possible combination of test results, as the data permit. These risk scores are obtained at time points, 0 (immediate), 1, 2, 3, 4, and 5 years.
Each risk estimate is presented with its corresponding standard error (SE) and 95% lower (LL95) and upper (UL95) confidence interval.
Test results are ordered chronologically; therefore, in each table, the leftmost column presents the oldest test result in the screening history of the patient while the rightmost column(s) (among the test results) displays the current test result. The total sample size (N) in each category and each screening result as a percentage (%) of total screened, the total number of patients informative in risk estimation (N Informative) are listed in the columns following the test results. Total number of observed CIN 2+, CIN 3+, and cancer cases are displayed together with the breakdown of prevalence, incidence, and unknown prevalence/incidence in the subsequent columns.
Following the risk estimates columns, recommended management and “Recommendation Confidence Score” (for more details about this score please refer to Egemen et al.) are listed. Sampling weights are used for the sample from which we obtained the risk estimates for the HPV genotyping test results, as explained in the article Demarco et al. The raw sample sizes (without the sampling weights) are presented at the rightmost columns of each table. The statistical methods for risk estimation are explained in the article Cheung et al.
Please note that test results are considered valid if they are documented in the medical record as a negative HPV test or cotest within an appropriate screening interval (approximately 5 years), or as a colposcopic examination <CIN2 within the past year.
The generation of these risk estimates was supported by the Intramural Research Program of the National Cancer Institute. The risk estimates are in the public domain in the United States of America and are made freely available elsewhere.
- Abnormal Screening Results
- Surveillance following results not requiring immediate colposcopic referral
- Receipt of colposcopy/biopsy results
- Surveillance visit following colposcopy/biopsy finding less than CIN 2 (no treatment)
- Follow-up after treatment for CIN 2 or CIN 3
Supplementary tables are available upon request. Please contact us via email.
2019 Guidelines Bibliography
Perkins RB, Fuzzell LN, Lake P, McIntyre M, Nayar R, Saraiya M, Loukissas J, Felder T, Guido RS, Vadaparampil ST. Incorporating Stakeholder Feedback in Guidelines Development for the Management of Abnormal Cervical Cancer Screening Tests. J Low Genit Tract Dis. 2020.
Demarco M, Egemen D, Raine-Bennett TR, Cheung LC, Befano B, Poitras NE, Lorey TS, Chen X, Gage JC, Castle PE, Wentzensen N, Perkins RB, Guido RS, Schiffman M. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020.
Egemen D, Cheung LC, Chen X, Demarco M, Perkins RB, Kinney W, Poitras N, Befano B, Locke A, Guido RS, Wiser AL, Gage JC, Katki HA, Wentzensen N, Castle PE, Schiffman M, Lorey TS. Risk Estimates Supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020.
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020.
Cheung LC, Egemen D, Chen X, Katki HA, Demarco M, Wiser AL, Perkins RB, Guido RS, Wentzensen N, Schiffman M. 2019 ASCCP Risk-Based Management Consensus Guidelines: Methods for Risk Estimation, Recommended Management, and Validation. J Low Genit Tract Dis. 2020.
Schiffman M, Wentzensen N, Perkins RB, Guido RS. An Introduction to the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis. 2020.
Clarke MA, Unger ER, Zuna R, Nelson E, Darragh TM, Cremer M, Stockdale CK, Einstein MH, Wentzensen N. A Systematic Review of Tests for Postcolposcopy and Posttreatment Surveillance. J Low Genit Tract Dis. 2020.
Clarke MA, Darragh TM, Nelson E, Unger ER, Zuna R, Cremer M, Stockdale CK, Einstein MH, Wentzensen N. Reporting and Assessing the Quality of Diagnostic Accuracy Studies for Cervical Cancer Screening and Management. J Low Genit Tract Dis. 2020.