Cervix
DCEG researchers conduct studies on cancers of the cervix, more commonly referred to as cervical cancer. The primary cause of cervical cancer is persistent infection with the human papillomavirus (HPV).
Studies include:
-
ASC-US/LSIL Triage Study (ALTS)
A study of the clinical management of low-grade cervical cytologic abnormalities
-
Biopsy Study to Improve Detection of Cervical Precancer
A collaborative study on colposcopic biopsy with aims to study cervical disease on the lesion level, optimize criteria for biopsy placement, and analyze the incremental benefit of taking multiple biopsies
-
Cervical Cancer Screening Among HIV-Infected Women in India
A study to evaluate a cohort of HIV-infected women using two novel and potentially sustainable, lower-cost tests for accurate screening for cervical cancer
-
Cervical Visualization Study
Cervical Visualization Study
-
Cancer Moonshot: HPV Vaccine Trial & Accelerated Cervical Cancer Control
DCEG researchers tested one-dose HPV vaccines and created new screening approaches.
-
Costa Rica HPV Vaccine Trial (CVT) and Long-Term Follow-Up
A randomized, controlled phase III trial of a vaccine to prevent human papillomavirus (HPV) 16 and 18 infections and their associated cervical lesions in Costa Rica (CVT)
-
Enduring Consensus Cervical Cancer Screening and Management Guidelines
A process to provide regular updates to the 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors
-
ESCUDDO Efficacy Trial: Evaluation of One or Two Doses of HPV Vaccine
Scientific evaluation of one or two doses of the bivalent or nonavalent prophylactic HPV vaccine
-
DCEG's Role in the Federal Cervical Cancer Collaborative
A partnership to control cervical cancer in safety-net settings of care.
-
Guanacaste HPV Natural History Study
A population-based natural history study of HPV and cervical neoplasia launched with Costa Rican colleagues in 1993.
-
Improvement of Risk-Informed Screening (IRIS)
A comprehensive evaluation of candidate biomarkers for use in cervical cancer screening and triage.
-
Methylation of Human Papillomavirus (HPV) and Cervical Cancer Risk
Studies of Human Papillomavirus (HPV) Methylation
-
HPV Natural History, Genomics and Risk Assessment
Studies of human papillomavirus (HPV) natural history, genomics and risk assessment that led to screening and clinical management.
-
Human Papillomavirus (HPV) Persistence and Progression Cohort (PaP Cohort)
A collaboration with Kaiser Permanente Northern California to create a repository of specimens
-
PRIMAVERA Immunobridging Trial
The trial is designed to determine if a single dose of HPV vaccine will elicit sufficient immune response to protect against targeted HPV infections.
-
PRISMA Efficacy Trial
PRISMA seeks to determine if one dose of HPV is sufficient to reduce the risk of HPV infection in young adult women, compared to a non-HPV vaccine
-
Risk Prediction Modeling for Cervical Cancer
Cervical cancer risk prediction incorporating clinical and laboratory covariates
-
STRIDES: Studying Risk and Improving Disparities of Cervical Cancer in Mississippi
The NCI is partnering with the University of Mississippi Medical Center to evaluate risk of cervical precancer and to study novel biomarkers.
-
Study to Understand Cervical Cancer Early Endpoints and Determinants (SUCCEED)
A study to comprehensively assess biomarkers of risk for progressive cervical neoplasia, and thus develop a new set of biomarkers that can distinguish those at highest risk of cervical cancer from those with benign infection
-
The Selfie Study
A study of novel biomarkers to detect cervical precancer from self-collected samples.
Research News
-

Nicolas Wentzensen Talks About Self-Collection in the NCI Cancer Currents Blog
Nicolas Wentzensen discusses the recent FDA approval of self-collection kits for use in clinics for cervical cancer screening. His interview was published in the NCI Cancer Currents Research Blog.
-
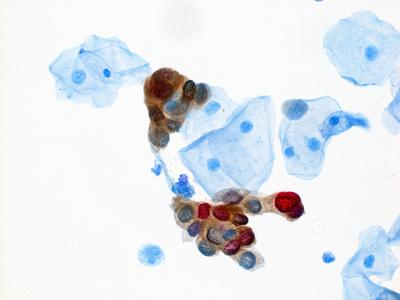
Enduring Guidelines: Methods and Dual Stain for Cervical Cancer Screening Triage
Continual improvement of cervical cancer screening and management to include new technologies and approaches requires a flexible approach to guidelines. A description of the Enduring Guidelines effort—methods and principles to ensure swift adoption of changes—and a review and decision on the first new technology to be added to the guidelines—dual stain cytology—were published in the Journal of the Lower Genital Tract Diseases.
-
Differences in Knowledge of HPV and the HPV Vaccine by Education, Race, and Ethnicity
Ms. Erica Stephens and Dr. Jaimie Shing used data from a nationally representative sample of U.S. adults to ascertain awareness of HPV and the HPV vaccine, as well as knowledge that HPV can cause cancers, by educational attainment, race, and ethnicity. They found profound disparities that signal the importance of continued education around HPV and the HPV vaccine.
-

Catch-Up HPV Testing May Help Prevent Cervical Cancer in Some Over 65
It may be worthwhile for some individuals between ages 65 and 69 to get tested for HPV, findings from a Danish study suggest. Specifically, the testing may help prevent cervical cancer among those who haven’t had cervical cancer screening for at least 5 years.
-
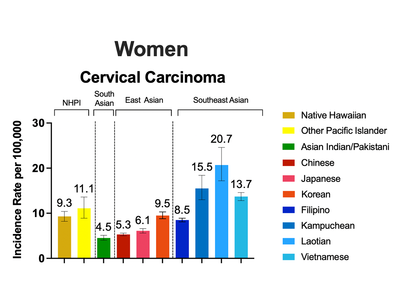
Disaggregating Data on Asian American and Native Hawaiian and Pacific Islander Populations by Ethnicity Reveals Disparities in HPV-Associated Cancers
An analysis led by Drs. Jacqueline B. Vo in the Radiation Epidemiology branch and Jaimie Z. Shing in the Infections and Immunoepidemiology Branch revealed disparities in incidence rates of human papillomavirus (HPV)-associated cancers in Asian American and Native Hawaiian and Pacific Islander populations when disaggregated by race and ethnicity.