Self Collection for HPV Testing to Prevent Cervical Cancer: New Guidelines Published
, by Jennifer K. Loukissas, M.P.P.
New guidelines for clinicians performing cervical cancer screening advise on management of HPV test results from self-collected vaginal samples. Self collection expands screening options and has potential to increase access for individuals who have never been screened or are not receiving adequate screening. The guidelines were published February 21, 2025, in the Journal of Lower Genital Tract Disease.
In 2024, the U.S. Food and Drug Administration approved primary human papillomavirus (HPV) testing of self-collected vaginal samples in healthcare settings.
HPV testing on self-collected samples has high agreement with testing on clinician-collected samples. At present, data on outcomes for patients screened with self-collected samples are mainly cross-sectional. Additional data are needed to evaluate longitudinal performance of HPV testing on self-collected samples. The guidelines advise:
- Self-collected vaginal specimens are acceptable for primary HPV screening of asymptomatic average risk individuals.
- For patients with prior abnormal screening test results, colposcopy, or treatment: Minimal data exist on use of self-collected vaginal specimens for patients in surveillance. Therefore, clinician-collected cervical specimens are preferred for these individuals.
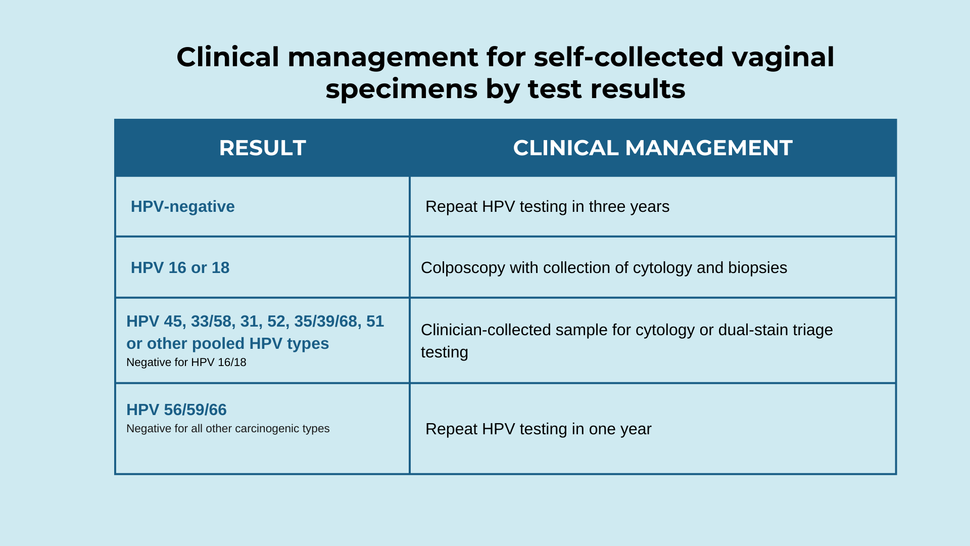
- Clinical management for self-collected vaginal specimens by test results are presented in the table below.
As the necessary cause of nearly all cervical cancers is persistent infection with a carcinogenic HPV type, the guidelines committee focused their comprehensive review of data on the performance of self-collected samples on HPV genotype agreement between self-collected vaginal and clinician-collected cervical specimens. The committee considered available data, input collected through a public comment period, and expert consensus. The guidelines were ratified through a vote by the Consensus Stakeholder Group.
Laboratory and clinical workflows will need to be modified to ensure adequate specimen processing and follow-up.
Reference
Wentzensen N et al. Self-Collected Vaginal Specimens for HPV Testing: Recommendations From the Enduring Consensus Cervical Cancer Screening and Management Guidelines Committee. JLGTD. 2025.