For DES-affected Individuals and Families
We hear from many DES-affected individuals and their families with requests to join the study, or questions about their health. The cohorts that make up the DES follow-up study are no longer recruiting participants. However, we will continue to post the research findings on this website.
The National Cancer Institute (NCI) does not have information about possible and yet unknown DES-associated conditions or complications in age groups (or generations) beyond what is discussed below and referenced in the linked scientific literature.
Resources for DES-exposed Individuals and Families
DES Action
NCI Fact Sheet on DES Exposure
NCI's Cancer Information Service (CIS) can help answer your cancer-related questions whether you are a patient, family or friend, health care provider, or researcher.
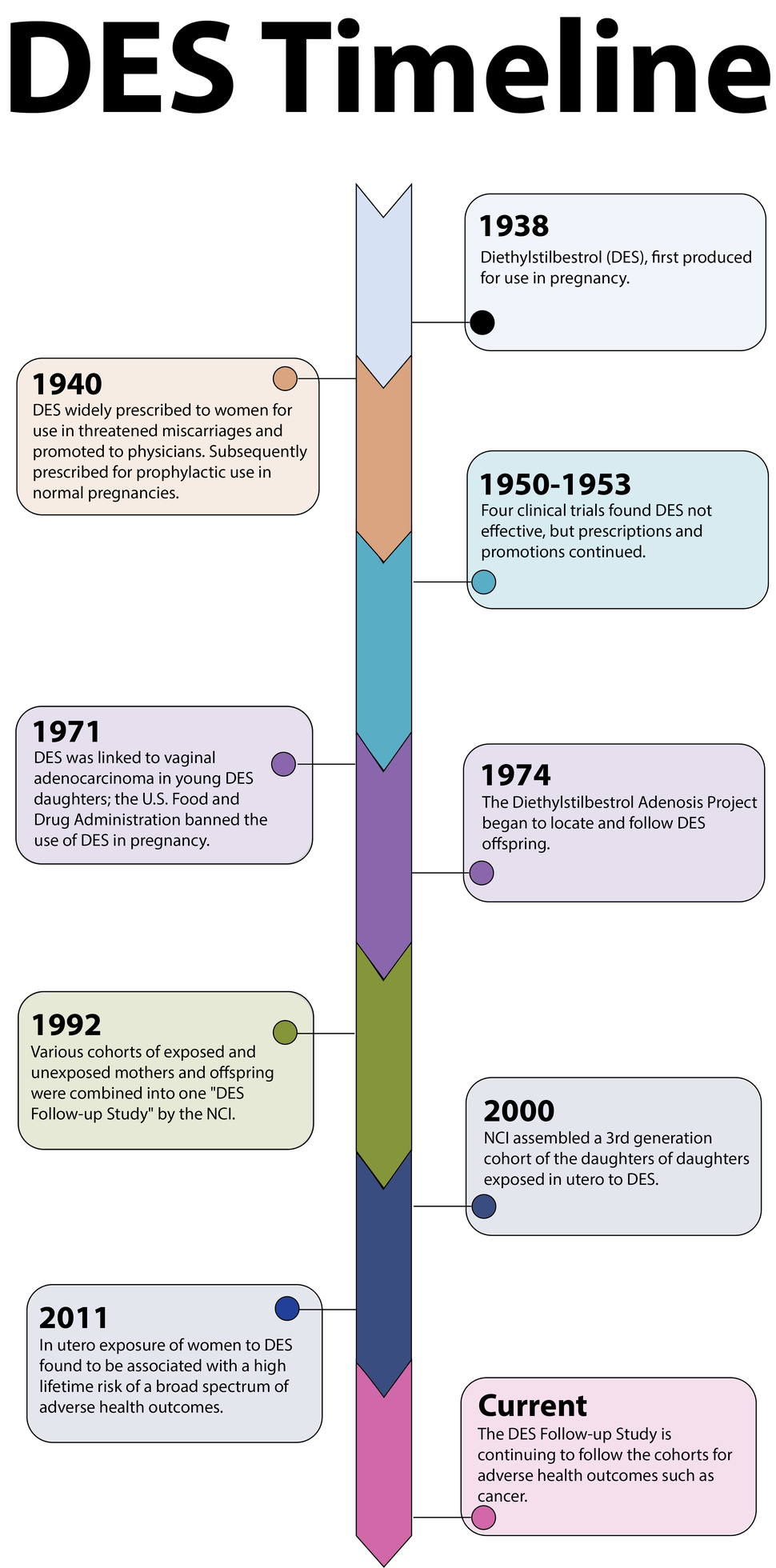
Background
Brief History of DES
Diethylstilbestrol (DES) is a known human carcinogen. It was the first estrogen pill, produced in 1938 and widely used from the mid-1940s until the early 1970s as a treatment for women who were at risk of miscarriages. It is estimated that millions of Americans (mothers, daughters, and sons) may have been exposed to DES.
Adverse Health Effects of DES
Following the 1971 paper, DES has been associated with several other health effects, including an increased frequency of problems of the reproductive tract, changes in the tissue of the vagina, infertility, and poor pregnancy outcomes in daughters.
About the NCI DES Follow-up Study
The study has documented the adverse health effects on women who took it during pregnancy, and their exposed daughters, sons, and granddaughters. As DES-exposed offspring are currently reaching the age when cancer rates begin to rise, it is important to continue to monitor the long-term risk of cancer and other adverse health outcomes in this unique population.
The NCI DES Follow-up Study is a nationwide research study following more than 21,000 women and men to learn as much as possible about the long-term health effects of DES exposure. It is the largest ongoing study on long-term health and DES exposure. Since 1992, NCI investigators and collaborators at five field study centers have actively investigated health outcomes related to DES exposure in three generations of DES-exposed and unexposed participants. It is the largest ongoing research study on long-term health and DES exposure. Leaders in DES research and education are responsible for the study and are dedicated to increasing scientific and medical knowledge about DES exposure. The research team includes physicians, epidemiologists, researchers, DES advocates, and educators.
The first generation (DES mothers) comprises women exposed during pregnancy. The second generation (DES daughters and sons) comprises women and men exposed to DES in utero. The third generation (granddaughters) comprises daughters of prenatally exposed women. Within each of these three generations, risks associated with exposure to DES are assessed by comparing health outcomes in the DES-exposed with those of the unexposed.
In addition to answering important health-related questions for the DES-exposed population, the NCI DES Follow-up Study also provides a model for the influence of prenatal hormonal exposure on disease risk, which has important implications for environmental exposures, particularly endocrine-disrupting chemicals.
Key Findings and Ongoing Research
See the full list of publications from the NCI DES Follow-up Study.
A landmark paper describing the adverse health effects observed in the NCI DES Follow-up Study, Adverse health outcomes in women exposed in utero to diethylstilbestrol, was reported in the New England Journal of Medicine in 2011. Those findings are described below.
Key Findings from the First Generation (Mothers)
Women who were exposed to DES while pregnant, when compared with the unexposed, have a modestly increased risk of breast cancer and breast cancer mortality. The increase in breast cancer mortality argues against the possibility that the increased incidence affecting the DES-exposed women was due to greater surveillance or more frequent mammographic screening. DES exposure during pregnancy was not related to other types of cancers.
Key Findings from the Second Generation (Daughters and Sons)
The NCI study has identified or confirmed increased risks of numerous adverse health outcomes in women exposed in utero to DES. These included elevated risks of infertility, spontaneous abortion, preterm delivery, loss of second-trimester pregnancy, ectopic pregnancy, preeclampsia, stillbirth, neonatal death, early menopause, grade 2 or higher cervical intraepithelial neoplasia, breast cancer at age 40 or more, and clear cell adenocarcinoma of the vagina. Risk of many of these conditions was higher among women with vaginal epithelial changes (adenosis).
NCI investigators observed excess risk of urogenital anomalies in the prenatally DES-exposed men, although these did not appear to affect fertility. A possible excess of testicular cancer was also noted.
Key Findings from the Third Generation (Granddaughters)
In 2000, the NCI Third Generation Study began actively enrolling and following the daughters of prenatally DES-exposed and unexposed women. Data arising from this study suggested the DES-exposed granddaughters may have an elevated risk for infertility, an outcome known to affect prenatally exposed women. The DES-exposed granddaughters also had a greater likelihood of amenorrhea (skipped periods) and menstrual irregularity. In addition, investigators noted an excess of ovarian cancer in the DES-exposed. Because this finding was based on only three ovarian cancer cases, it must be considered tentative. Given their young age, the third generation women have not completed their reproductive years and remain at relatively low risk for most adverse health outcomes, underscoring the importance of continued follow-up.
Active follow-up of the third-generation women is limited to the daughters of prenatally exposed and unexposed women. To investigate important outcomes in the larger group of grandchildren, which includes the daughters of prenatally exposed and unexposed men, as well as the sons, NCI investigators asked second-generation women to report cancer diagnoses affecting their children. This approach supported the possibility of an excess of ovarian cancer, as seen in the actively followed DES-exposed granddaughters, but has not shown an increased risk of cancers in the DES-exposed grandsons.
Ongoing Research and Recent Findings
We are continuing to study new information on prenatal DES exposure and the following health outcomes and biomarkers:
- Benign breast disease
- Health effects among women whose mothers were exposed to DES in utero (the Third Generation Study or the granddaughters’ study)
- DNA methylation patterns, in collaboration with researchers at the National Institute of Environmental Health Sciences
- Genetic markers and hormone metabolism in DES-exposed daughters, in collaboration with scientists from Boston University and the University of Chicago (on hold due to the pandemic)
Recent Findings
High-Grade Squamous Intraepithelial Lesions (HSIL)
The age-adjusted risk of high-grade squamous intraepithelial lesions (HSIL) of the lower genital tract (cervical and vaginal) among DES-exposed women was higher than among DES-unexposed through age 44 but not age 45 years or older. Elevated HSIL risk remained higher in DES-exposed women after accounting for the frequency of cervical cancer screening. These data confirm the appropriateness of more frequent screening among DES-exposed women through age 44. Whether those aged 45 years or older should continue to have increased screening will require careful weighing of possible risks and benefits.
Troisi R, et al. An Update on Prenatal Diethylstilbestrol Exposure and High-Grade Squamous Intraepithelial Lesions of the Lower Genital Tract. Obstetrics & Gynecology. 2024
DNA Methylation Patterns & Estrogen Metabolism
An analysis of blood DNA methylation in women exposed and unexposed to DES in utero using samples from 60 women (40 exposed and 20 unexposed) in the NCI DES Follow-up Study and 199 women (99 exposed and 100 unexposed women) in the NIEHS Sister Study Cohort examined nine candidate genes related to cell proliferation and differentiation. In six of the genes, blood DNA methylation levels were statistically significantly associated with prenatal DES exposure, suggesting that in-utero DES exposure may be associated with differential blood DNA methylation levels, which could mediate the increased risk of several adverse health outcomes observed in exposed women. These findings need further evaluation using larger data sets.
Among a subset of participants, preliminary data suggest that postmenopausal women prenatally DES exposed may have estrogen metabolism patterns that differ from unexposed women. The investigators observed relatively less 2 than 16 pathway estrogen metabolism among exposed compared to unexposed women. Lower 2 pathway metabolism has been associated with increased postmenopausal breast cancer risk and could potentially offer a partial explanation for the modest increased risk observed for prenatally DES-exposed women.
Troisi R et al. Estrogen Metabolism in Postmenopausal Women Exposed ;In Utero to Diethylstilbestrol. Cancer Epidemiol Biomarkers Prev. 2018.
Bodelon C et al. In utero exposure to diethylstilbestrol and blood DNA methylation in adult women: Results from a meta-analysis of two cohort studies. Environmental Research. 2023.
Cardiovascular Outcomes
Women exposed to DES in utero were at elevated risk for coronary artery disease and myocardial infarction, but not for stroke. These elevated risks appeared to be independent of established cardiovascular disease risk factors.
Troisi R et al. A Prospective Cohort Study of Prenatal Diethylstilbestrol Exposure and Cardiovascular Disease Risk. J Clin Endocrinol Metab. 2018.
Pancreatic Disease
Prenatal DES exposure may increase the risk of pancreatic disorders, including pancreatitis in women and men. The data suggested elevated pancreatic cancer risk in DES-exposed women, but not in exposed men.
Troisi R et al. Prenatal diethylstilbestrol exposure and risk of diabetes, gallbladder disease, and pancreatic disorders and malignancies
History of DES Research at the NCI
The NCI study was funded through the DES Research and Education Act of 1992.
Congress passed two bills requiring research on DES exposure. The National Institutes of Health (NIH) sponsored workshops on DES in 1992 and 1999 and made the following recommendations:
- Continue to monitor the people participating in studies of DES for cancer risk, particularly among daughters as they approach menopause, and sons exposed to DES;
- Assess the impact of and risks associated with exposure to other hormones such as hormone replacement therapy in DES-exposed mothers and daughters;
- Study whether DES-exposed women and men will develop non-reproductive conditions.
The Five Cohorts that Make up the DES Follow-up Study
Participants were initially drawn from eight different medical centers and consisted of five previously studied cohorts:
DESAD (Diethylstilbestrol Adenosis Project) Cohort
The large DESAD cohort, first assembled in 1975, enrolled more than 4,000 prenatally DES-exposed and 1,000 unexposed women identified through Baylor College of Medicine, Gundersen Clinic, Massachusetts General Hospital, the Mayo Clinic, and the University of Southern California. The unexposed group comprised sisters of exposed participants and other women who were not related to the exposed participants.
The primary goal of DESAD was to provide yearly clinical examinations for the daughters to identify women with vaginal adenosis, a precursor for vaginal clear cell adenocarcinoma, and assess reproductive problems. Exposed and unexposed daughters underwent identical examinations through l983 and completed yearly questionnaires from 1984 to 1989 to document their reproductive and medical history. DESAD participants with documented DES exposure were included in the NCI DES Follow-up Study and comprise the largest of the five NCI cohorts.
Women’s Health Study Cohort
The goal of the Women's Health Study (WHS), which began in 1981, was to assess breast cancer risk in women who were exposed to DES while pregnant. More than 3,000 women exposed to DES at any time during pregnancy and 3,000 unexposed women were identified through a review of 1940-1960 obstetrics records at the Boston Lying-in Hospital, the Mayo Clinic, Dartmouth Medical School (Mary Hitchcock Hospital), and a private obstetrical practice in Portland, Maine. These women completed questionnaires during the 1980s, and medical records were collected to confirm cancer diagnoses.
The prenatally exposed and unexposed daughters and sons of these women had not been studied previously. When the WHS cohort joined the NCI study, the field study centers expanded enrollment to include more than 1,000 daughters and 1,000 sons of WHS study participants.
Mayo Clinic Cohort
The Mayo Clinic Cohort has the largest number of sons included in the DES Follow-up Study. Participants were identified by reviewing the obstetrics records of women who gave birth at Mayo Clinic hospitals between 1939 and 1961. Men who were prenatally exposed to DES and others who were not exposed were asked to participate. The medical records of these sons were studied to determine if DES exposure was related to an increased risk of infertility, problems with the genitals or urinary tract, and cancer. About one-third of both the exposed and unexposed sons also had a physical examination at the Mayo Clinic during the early 1980s.
Dieckmann Cohort
Between 1950 and 1952, pregnant women were enrolled in the Dieckmann Study, a clinical trial conducted at the University of Chicago to determine whether DES was effective for preventing pregnancy loss. In the mid-1970s, trial participants were located and queried for interim health outcomes. Around the same time, investigators attempted to enroll the daughters and sons of these women; those who participated were physically examined and queried for reproductive tract and genitourinary anomalies.
Horne Cohort
The Horne cohort includes mothers who were treated with DES by Dr. Herbert Horne, an infertility physician in Boston, and their exposed and unexposed children. Beginning in 1971, Dr. Horne periodically sent questionnaires to these participants.
For Participants: Update your Contact Information
Please contact your Research Center Coordinator (see below) with any changes to your name, address, telephone number, or email address. If you do not know which center to contact, choose the one located closest to you and that coordinator will be able to help you.
Baylor College of Medicine
Toll-Free (855) 443-0089
FollowUpStudy@westat.com
Boston University
Study Coordinator - Hannah Lord
(617) 206-6220
desstudy@bu.edu
Geisel School of Medicine at Dartmouth
Prinicipal Investigator - Linda Titus, Ph.D., M.A.
linda.titus@dartmouth.edu
Mayo Clinic
(507) 284-2747
desstudy@mayo.edu
The University of California at Los Angeles Medical Center
Toll-Free (855) 443-0089
FollowUpStudy@westat.com
University of Chicago
Study Coordinator - Minji Kang
(773) 702-6671
chicagodesprogram@babies.bsd.uchicago.edu
University of Massachusetts Medical School Boston, Massachusetts
(507) 284-2747
desstudy@mayo.edu