Understanding the Role of Physical Activity, Obesity, and Diet in Cancer: Profile of Tenure-track Investigator Steven Moore
, by Justine E. Yu, Ph.D.
Tenure-track investigator Steven C. Moore, Ph.D., M.P.H., has built a multidisciplinary research program in the Metabolic Epidemiology Branch (MEB) focused on understanding the contributions of physical activity, obesity, and diet to human cancer and overall health.
Exploring the Links Between Exercise and Longevity
The U.S. physical activity guidelines recommend adults to engage in a minimum of 150 minutes per week of moderate-intensity, or 75 minutes per week of vigorous-intensity aerobic physical activity or an equivalent combination. However, more than 80% of U.S. adults fail to meet these targets, contributing to the global burden of premature mortality and cancer. Additionally, the changing culture has resulted in tremendous increases in sedentary behavior—time spent sitting during the workday or at home watching television. “We know now that the combination of obesity and sedentary behaviors contributes definitively to cancer risk,” Dr. Moore said. “However, ten years ago, the association of physical activity and cancer was less established.”
Prior studies have demonstrated the association of physical activity with a lower risk of all-cause mortality, but until recently, the years of life expectancy gained over different levels of activity was unclear. To quantify the level of physical activity associated with maximum health benefits, Dr. Moore and colleagues pooled data from various populations to describe the effects in terms of years of life gained (Moore SC et al, Plos Med 2012). They observed an increase in life expectancy with increasing leisure time physical activity. Further, they observed surprisingly large benefits at even very low levels of activity: the exercise equivalent of brisk walking just 10 minutes a day was associated with a gain of 1.8 years of life relative to no leisure-time physical activity. Activity at or above the recommended levels was associated with more years of life gained, though benefits diminished for activity beyond four times the recommended minimum.
Expanding on Physical Activity and Cancer Risk
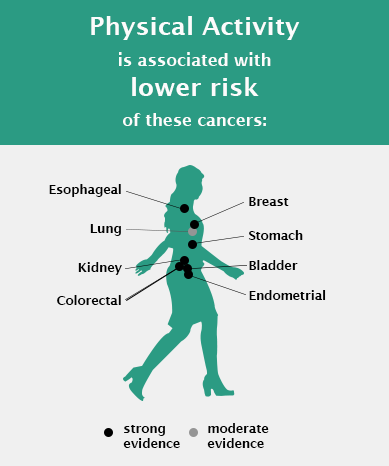
After working to define the relationship of physical activity and overall health, Dr. Moore and colleagues examined the effects of physical activity in relation to cancer risk and prevention in the largest study to date, evaluating 26 types of cancer among 1.4 million people (Moore SC et al, JAMA IM 2016).
“Previously there was strong evidence linking physical activity to lowered risk of breast, colon, and endometrial cancers, which only covers approximately 25% of total cancer incidence,” explained Dr. Moore. “Our study expanded the current knowledge on the range of cancer sites associated with physical activity.”
They found that high levels of leisure time physical activity were associated with lower risks for ten additional cancer types, including esophageal, liver, lung, kidney, gastric, head and neck, and bladder cancers, as well as myeloid leukemia and multiple myeloma. The 2018 U.S. Physical Activity Guidelines Advisory Committee (PAGAC) report promptly incorporated these findings as evidence to support the link of physical activity with lower risk of eight different types of cancer (Piercy KL et al, JAMA 2018).
Recently, Dr. Moore and colleagues extended their analysis to describe the amount of physical activity needed to achieve these health benefits and confirmed in prospective data the suitability and protective effect of physical activity recommendations (7.5-15 MET hours/week) set by the 2018 PAGAC (Matthews CE et al, JCO 2019). For some cancers, though, they found potential benefits even beyond this level. “We previously thought the benefits of physical activity plateaued after 15 MET hours/week however, our evidence suggests that activity levels beyond that threshold are associated with continued risk reduction for colon, breast, endometrial, head and neck, and esophageal cancers,” said Dr. Moore.
In addition, he and research analyst, Kaitlyn M. Mazzilli, M.P.H., are investigating specific types of physical activity in relation to cancer risk. In a recent study, they found weight training was significantly associated with reduced risk for colon cancer and but no significant effect on risk for nine other cancers (Mazzilli KM et al, Med Sci Sports Exerc 2019). This suggests the need for more research examining the different effects of weight and aerobic training on cancer risk. Currently, Dr. Moore and Ms. Mazzilli are evaluating stair climbing in relation to health outcomes.
Research efforts over the last ten years by Dr. Moore, Charles E. Matthews, Ph.D., senior investigator in MEB, and others studying exercise oncology, were highlighted in the 2018 Report from the American College of Sports Medicine Roundtable on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. The report encourages health care and fitness professionals to recommend physical activity for cancer prevention and to provide exercise prescriptions that best meet the needs, preferences, and abilities of individuals living with and beyond cancer.
Metabolomics: Breaking Ground on the Role of Obesity in Cancer
Over 30 years, the prevalence of obesity has steadily risen in the U.S. While studies have established that obesity is associated with increased risk of many cancers, the biological mechanisms are not well understood.
“Many cancer researchers are now investigating how metabolism affects the development, etiology, and pathogenesis of cancer,” said Dr. Moore. To enhance understanding of the impact of metabolism and obesity on cancer risk, Dr. Moore developed a research program focused on metabolomics—the study of metabolic compounds and their relationship to underlying human physiology.
Using metabolomics, Dr. Moore and colleagues examined how metabolites relate to body mass index (BMI) in three different cohorts, and found that, out of 317 blood metabolites studied, 37 were significantly associated with BMI (Moore SC et al, Metabolomics 2014). These metabolites constitute the foundation of a BMI-related metabolic profile.
Expanding on that work, Dr. Moore then identified four BMI-associated metabolites that were associated with future breast cancer risk in postmenopausal women (Moore SC et al, JNCI 2018). Three of them—allo-isoleucine, 2-methylbutyylcarnitine, and 3-methylglutaryl-carnitine—are involved in a single metabolic pathway that breaks down branched-chain amino acids. When the normal pathway is disrupted, these metabolites accumulate in the blood and may provide amino acid building blocks needed for cancer cells to proliferate. The fourth, 16α-hydroxy DHEA 3-sulfate, a rarely studied precursor to estradiol, may independently contribute to breast cancer risk.
Ongoing research with post-doctoral fellow Kathleen M. McClain, Ph.D., M.S., further explores these BMI-related metabolites to determine which component of BMI is responsible for associations: fat mass or lean mass. In a study where all participants had their body composition measured by whole body dual X-ray absorptiometry, Drs. Moore and McClain are finding that increased lean mass, not just increased fat mass, explains some of the detrimental metabolic perturbations associated with obesity.
Leveraging Metabolomics to Identify Diet Biomarkers of Cancer Risk
Historically, studies on the relationship between diet and health have relied on food frequency questionnaires, a self-reported measure of diet, to assess food and nutrient intake. Metabolomics provides a way to measure dozens, if not hundreds, of dietary biomarkers simultaneously, which can in turn be used as objective measures of diet in cancer etiology studies.
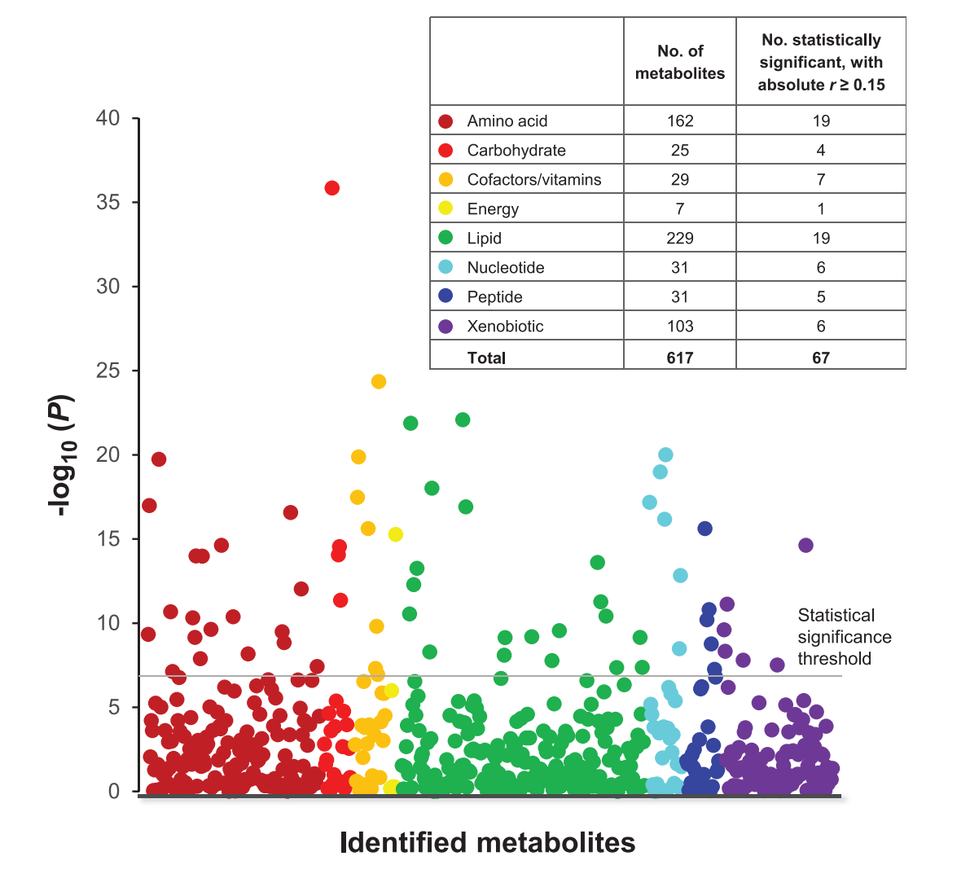
Dr. Moore and Mary Playdon, Ph.D., formerly a post-doctoral fellow in DCEG and now an assistant professor in the University of Utah, have conducted extensive exploratory studies to determine how well metabolomics is likely to perform for the identification of diet biomarkers. One of their studies found that 113 of 617 blood metabolites were substantially correlated with at least one of 55 different foods and beverages measured by self-report (Playdon MC et al, Am J Clin Nutr 2017). Many of the correlations appeared to reflect metabolites that were components of the food or beverage in question, supporting use of the metabolites as diet biomarkers, while others appeared to reflect how food was processed or metabolized.
Drs. Moore and Playdon are currently validating these findings in a study of 150 women who participated in a 14-day feeding trial that provided them with their usual foods and beverages under highly controlled conditions allowing precise measurement of their entire diet. By performing metabolomics analyses on the blood and urine samples collected at the end of the study, they will be able to benchmark metabolites against a gold-standard measure of diet. “This study involves women consuming their normal diet but with all food and beverages weighed to the gram. This is the perfect study for testing how well a biomarker is likely to perform as a measure of diet in a population-setting,” Dr. Moore said. “For biomarkers that validate, we can then carry them forward to large-scale population studies to complement our self-report measures of diet and to explore new dimensions of diet, such as food processing or individual-specific food metabolism.”
Using the Power of Consortia with COMETS
While metabolomics provides vast opportunities to delve deeper into nutritional epidemiology, individual studies are limited in size and often lack demographic and socioeconomic diversity. To address these issues for health research, Dr. Moore co-founded and helps to lead the Consortium of METabolomics Studies (COMETS) with intramural collaborators and researchers at extramural institutions. COMETS began as a meeting of representatives from 17 international prospective cohorts in 2014 and has grown to include 65 cohorts comprised of over 110,000 research participants. To facilitate consortium-based analyses, Dr. Moore and colleagues developed COMETS Analytics, a web-based tool to streamline and standardize the meta-analysis of the metabolomics consortium data. With the methodological foundation in place, ongoing studies by Dr. Moore and Rachel Kelly, Ph.D., of Harvard University, are exploring the relationship between BMI and metabolites within COMETS.
“There is still much to learn about how physical activity, diet and obesity play into cancer risk. Metabolomics can help us address long-standing research gaps within epidemiology on these topics,” said Dr. Moore.