COVID-19 Research: Cancer Screening, Mosaicism and Infection, Seroprevalence Data Visualization
, by Sharon A. Savage, M.D., and Jennifer K. Loukissas, M.P.P.
Scientists and clinicians across DCEG continue to apply their expertise to the study of the SARS-COV-2 pandemic and to volunteer their time to support the health of their colleagues, the NIH community, and others. Below is a summary of the work since our last update.
Ongoing Surveillance of Population-level Effects of the COVID-19 Pandemic on Mortality
Experts in descriptive epidemiology from across the Division continue to monitor the devastating effects of the global COVID-19 pandemic on mortality in the U.S. In the most recent analysis, the team reported on the death toll on Black, American Indian/Alaska Native, and Latino individuals. They concluded that the pandemic caused more deaths by population size, both directly and indirectly, in these groups compared with White or Asian individuals. The findings came from a large surveillance study and was carried out in collaboration with the National Institute for Minority Health and Health Disparities and the Pacific Institute for Research and Evaluation.
SeroHub Matures to a Robust System to Track the Pandemic in the U.S.
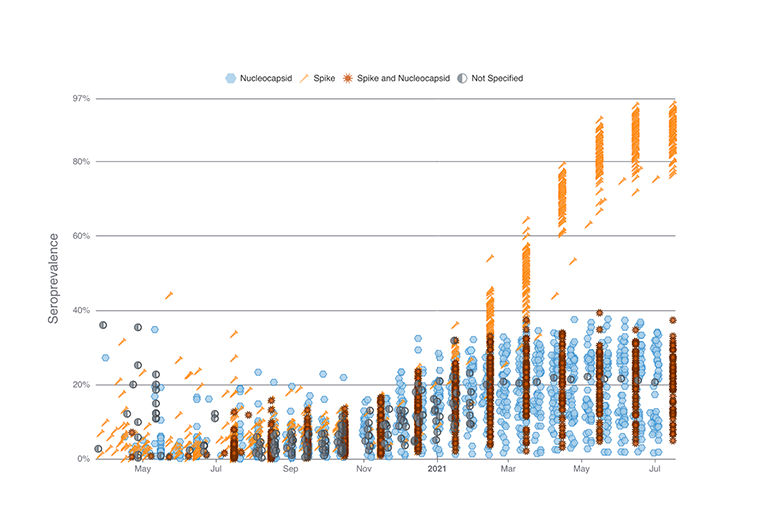
The web-based system SeroHub has amassed results from more than 70 seroprevalence studies that use blood tests to determine the proportion of people with antibodies to the SARS-CoV-2 virus. Prior to widespread vaccination, a positive antibody test indicated that a person had been infected with SARS-CoV-2, including asymptomatically. With the emergence of widespread vaccination, antibody tests distinguishing vaccination from natural infection can also be used to track vaccination progress in the population.
SeroHub has data from studies conducted in all 50 states, the District of Columbia, and Puerto Rico. Users can identify and explore these studies by time, geography, population, and serology test characteristics. Additionally, data visualizations have been developed to allow users to explore trends in SARS-CoV-2 seroprevalence over time, from the start of the pandemic to the present day. Trends can be viewed using all of the abstracted data or among particular states or study populations.
By bringing together results from different studies, clear patterns emerge. For example, although SARS-CoV-2 seroprevalence increased universally over the course of the pandemic, large differences were observed between states and study populations. A key focus of future work in SeroHub will be on vaccination in order to quantify geographic and population differences in vaccine uptake. Seroprevalence studies examining the impact of vaccination are just beginning to be published. SeroHub will allow users to track these studies and identify disparities.
A Lag in Cancer Screenings During the Pandemic Raises Questions about Prevention
While routine preventive medical care was on pause during the height of the pandemic, the U.S. and other countries experienced a drop in the diagnosis of incident cancers. Consequently, a number of scientific questions have arisen, prompting the President’s Cancer Panel to take up the topic of early detection approaches for four cancers—lung, colorectal, cervical, and breast cancers—in the fall of 2020. Nicolas Wentzensen, M.D., Ph.D., M.S., senior investigator in the Clinical Epidemiology Unit, Clinical Genetics Branch (CEU/CGB) co-led the group focused on cervical cancer.
Through these meetings, stakeholders across the spectrum of the National Cancer Program identified barriers to improving cancer screening as well as opportunities for overcoming them. The Panel met with key innovators to help develop and implement improvements in the areas of public health and community engagement; data sharing and professional training; as well as remote care and insurance coverage. For cervical cancer, in particular, the work of Dr. Wentzensen and colleagues has informed new clinical management and screening guidelines to accelerate control of cervical cancer. Important innovations that can play an important role in addressing health disparities and a screening backlog related to the pandemic include HPV-based primary screening and self-sampling, which can provide accurate screening without the need for patient-provider interactions in clinic settings, increasing the reach of screening while reducing the risk of SARS-CoV-2 exposure.
The panel’s report is expected later in 2021.
Clinical Validation of Isothermal Amplification-based SARS-COV-2 Detection Assays
As the U.S. and other countries gradually return to cancer screening and other elective procedures, providers and patients must undergo frequent SARS-CoV-2 testing, which range from rapid antigen tests with limited sensitivity to highly sensitive but time-intensive PCR-based tests. Evaluating rapid and accurate SARS-CoV-2 tests is particularly important for low and middle-income countries where COVID vaccinations still remain out of reach. Investigators in CEU/CGB applied their experience in testing and validating screening assays for cervical cancer to isothermal amplification-based assays (IAB) for SARS-CoV-2 with the hope that this rapid test would have sufficient sensitivity to identify patients who may spread SARS-CoV-2.
Dr. Wentzensen, Mark Schiffman, M.D., M.P.H., CEU/CGB senior investigator, and Kanan T. Desai, M.D., M.P.H., CEU/CGB staff scientist in collaboration with Basic Health International and Rutgers New Jersey Medical School, conducted a large-scale international multi-centric clinical evaluation of two IAB tests using different sampling strategies to identify infectious subjects with high viral loads before they undergo cancer screening or other outpatient procedures.
The team found that IAB assays have good sensitivity and high specificity, particularly for nasopharyngeal and mid-turbinate samples. While they concluded that IAB assays could reduce the risk of SARS-COV-2 transmission in outpatient clinics and allow for safer reopening of essential medical services, variation of performance between study sites and assays highlights the need for site-specific clinical validation of emergency-use-approved assays before clinical adoption. These findings were recently published.
Applying Expertise on Mosaicism to Infection Outcomes
Mitchell Machiela, Sc.D., M.P.H., Earl Stadtman Investigator in the Integrative Tumor Epidemiology Branch (ITEB), Shu-Hong Lin, Ph.D., ITEB postdoctoral fellow, and colleagues, examined the association between mosaic chromosomal alterations (mCAs) in immune cells and increased predisposition to infections. Their findings suggest that mCAs are linked to increased susceptibility to respiratory system infections, including COVID-19.