Overview
DCEG investigators are studying risk of anal cancer and biomarkers for anal cancer screening in a cohort of 363 men with HIV who have sex with men who were enrolled in the Kaiser Permanente Northern California (KPNC) health maintenance program.
Study Team
Lead Investigator
Nicolas Wentzensen, M.D., Ph.D., M.S.
Clinical Genetics Branch
Additional DCEG Investigator
Philip E. Castle, Ph.D., M.P.H.
Clinical Genetics Branch
Partner Organization
Kaiser Permanente Northern California (KPNC)
Background & Purpose
Men with HIV who have sex with men are at substantially increased risk of anal HPV infections and anal cancer compared to the general population. Anal cancer screening has been proposed for this population, but systematic evaluation of different screening tests has been lacking. Currently, anal cytology and high-resolution anoscopy are the main approaches used for anal cancer screening in high-risk populations. Given the similarities between cervical and anal carcinogenesis and natural history, there is a strong argument to evaluate new biomarkers, like HPV testing and genotyoping, mRNA testing, and p16/Ki-67 dual stain that have shown promise in cervical cancer screening. The goal of the Anal Cancer Screening Study is to evaluate risk factors associated with anal disease stages and biomarkers for anal precancer screening.
See all DCEG research on anal cancer and infectious agents.
Study Design
In collaboration with clinicians from KPNC, DCEG investigators established a cohort of 363 men with HIV aged 18 years and older who have sex with men and were undergoing anal cancer screening and high-resolution anoscopy (HRA) at the Anal Cancer Screening Clinic at KPNC from August 2009 to June 2010. Under written, informed consent, participants completed a self-administered risk factor questionnaire and underwent two anal specimen collections, using liquid-based cytology medium, as well as a digital rectal exam and HRA with target biopsies of suspicious lesions. Biopsy specimens were reviewed by KPNC pathologists, according to clinical practice. Participants were followed annually for two years to collect follow-up clinical data related to outcomes. Passive follow-up continued for over 5 years after the active follow-up period was completed.
Study Results & Select Publications
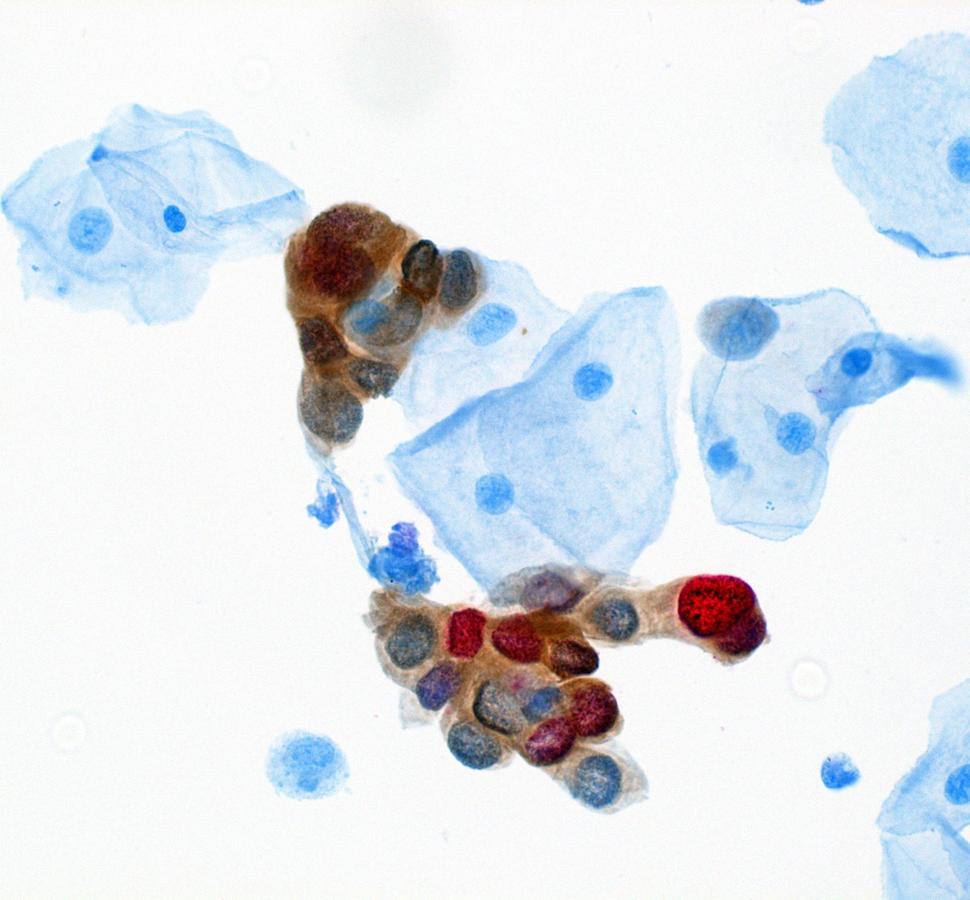
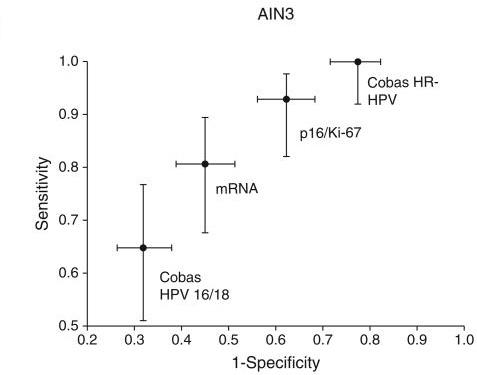
DCEG investigators have evaluated risk factors for transitions from anal HPV infection to anal precancers. Further, they evaluated baseline and longitudinal clinical performance of several biomarkers for anal precancer detection in this study, including carcinogenic HPV DNA in aggregate and individual carcinogenic HPV genotypes; HPV mRNA expression for certain high risk HPV types; p16/ Ki-67 “dual stain” immunocytochemical staining (both manual and automated evaluation); and liquid based anal cytology. Subsequently, DCEG investigators evaluated a new AI-based dual stain cytology assay which showed good performance for anal cancer screening. Stored biospecimens are available for biomarker testing when new assays become available, such as HPV methylation assays that have shown promise for detection of cervical precancers.
View publications related to the Anal Cancer Screening Study.