Anus
Overview
DCEG researchers conduct studies on anal cancers, the majority of which are caused by persistent infection with human papillomavirus (HPV), with an emphasis on populations at higher risk, such as people with HIV.
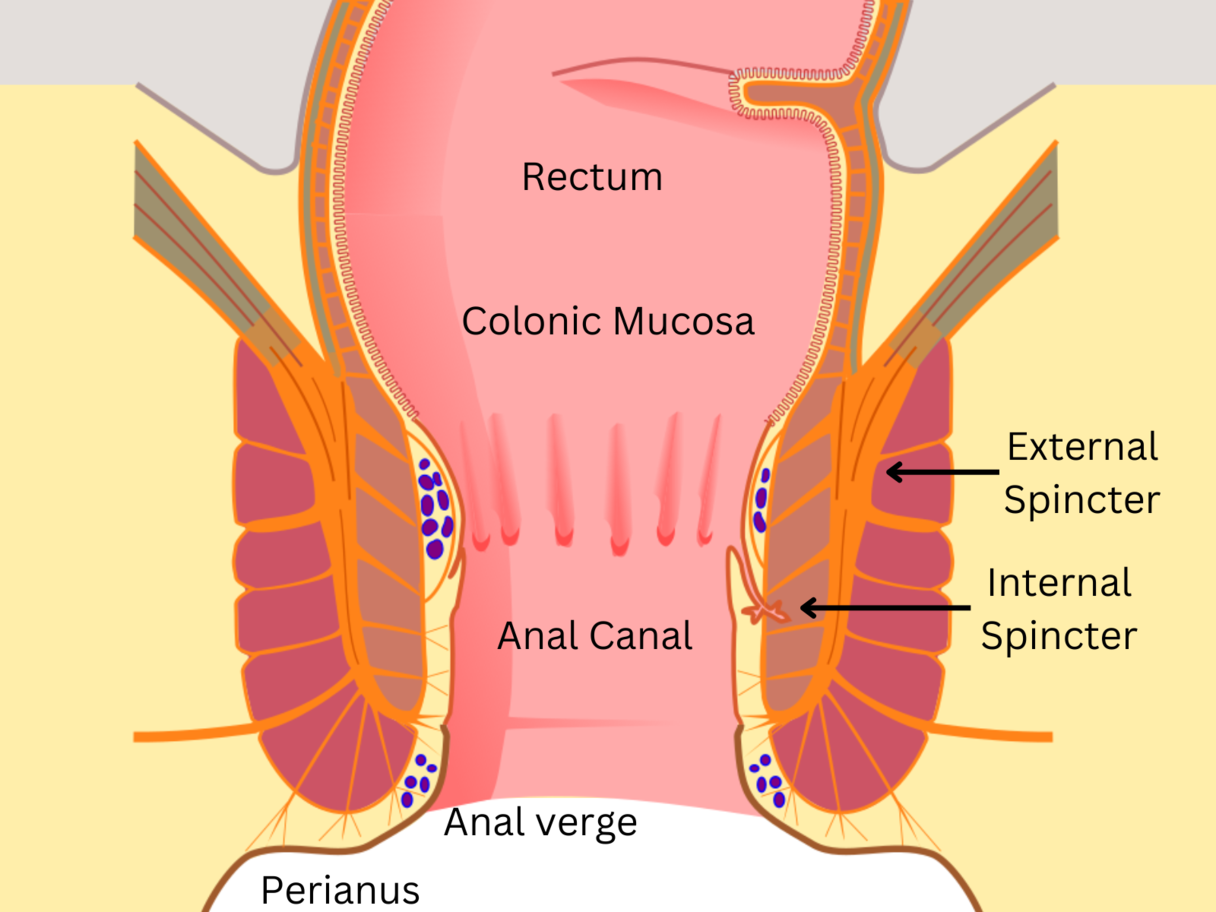
Anal anatomy.
Background & Purpose
In recent years, both the number of cases of anal cancer and deaths from the disease have dramatically increased in the U.S. According to the NCI Surveillance, Epidemiology, and End Results (SEER) Program, the overall incidence rates of anal cancer increased by 2.2% per year a between 2012 and 2021, and anal cancer mortality rates increased by 5.1% each year between 2013 and 2022. In 2024, the International Anal Neoplasia Society (IANS) published international consensus guidelines for anal cancer screening in certain groups with elevated risk. While these guidelines represent a critical foundation for anal cancer prevention, many knowledge gaps exist and future studies are needed to address pressing questions related to the harms and benefits of different screening approaches, implementation issues regarding HRA capacity and resource utilization, the performance of emerging technologies (i.e., p16/Ki-67 dual stain and extended HPV genotyping) for anal cancer screening and management, and the impact of HPV vaccination on screening performance.
DCEG research aims to quantify anal cancer risk, test the efficacy of the HPV vaccine on anal cancer prevention, and evaluate anal cancer screening tests and biomarkers to inform screening guidelines. This research is conducted by investigators in the Clinical Epidemiology Unit of the Clinical Genetics Branch and the Infections and Immunoepidemiology Branch.
Research Studies
Anal Cancer Screening Study
DCEG investigators are studying risk of anal cancer in HIV-positive men who have sex with men in a cohort of 370 HIV-positive men through the Kaiser Permanente Northern California health maintenance program.
Principal Investigator: Nicolas Wentzensen, M.D., Ph.D., M.S.
Branch: Clinical Genetics Branch
Collaborating Organization: Kaiser Permanente Northern California
Anal Cancer Etiology and Screening (ACES) Study
Researchers at NCI and Mount Sinai are evaluating the performance of cytology and HPV-related biomarkers for detection of anal precancer among individuals undergoing high resolution anoscopy through the Anal Cancer Prevention Program at Mount Sinai.
Principal Investigator: Nicolas Wentzensen, M.D., Ph.D., M.S.
Branch: Clinical Genetics Branch
Collaborating Organization: Icahn School of Medicine at Mount Sinai
The Costa Rica HPV Vaccine Trial
The HPV vaccine has the potential to prevent infections at all mucosal epithelial surfaces, including the anus. In the Costa Rica HPV Vaccine Trial, researchers study the long-term risk of anal cancer after HPV vaccination.
Principal Investigator: Aimée R. Kreimer, Ph.D.
Branch: Infections and Immunoepidemiology Branch
PRISMA Efficacy Trial
Researchers are conducting the PRISMA Trial to see if one dose of HPV vaccine is sufficient to reduce the risk of cervical HPV infection in young adult women. In addition to studying infection at cervical sites, the researchers will also investigate one dose HPV vaccine protection against oral and anal persistent HPV 16/18 infections.
Principal Investigator: Dr. Kreimer
Branch: Infections and Immunoepidemiology Branch
HIV/AIDS Cancer Match Study
DCEG investigators quantify anal cancer risk in people with HIV using data from the HIV/AIDS Cancer Match Study.
Principal Investigators: Eric A. Engels, M.D., M.P.H., and Meredith Shiels, Ph.D., M.H.S.
Branch: Infections and Immunoepidemiology Branch
Select Research Findings
Research using data from the HIV/AIDS Cancer Match Study has increased our understanding of anal cancer risk and mortality for people with HIV, showing that men who have sex with men diagnosed with AIDS have the highest 10-year cumulative risk for anal cancer. Additionally, the first study to report sex-specific anal cancer mortality for people with HIV found that both men and women with anal cancer and HIV have a higher risk of death compared to those without HIV.
Research from the Anal Cancer Screening Study has provided important data on the performance of anal cytology and HPV testing as well as more novel biomarkers like p16/Ki-67 (manual and automated) for anal cancer screening. Data from this study has been included in the evidence supporting the IANS screening guidelines.