Kidney Cancer
Serum PFAS Concentrations and Risk of Kidney Cancer
Higher kidney cancer incidence and mortality have been observed among individuals with high PFOA exposures from employment in a PFAS-producing chemical plant or residence in the surrounding community with contaminated drinking water. However, prospective studies had not assessed yet the relationship between PFOA and kidney cancer risk at levels of exposure comparable to those seen in the general population, and associations between other PFAS and risk of kidney cancer have not been evaluated yet. To address these questions, OEEB investigators conducted a nested case-control study investigating the risk of renal cell carcinoma (RCC, the most common form of kidney cancer) in relation to concentrations of PFOA and seven other PFAS measured in banked serum specimens within the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, a large U.S. population-based cohort. The investigation included 324 RCC cases diagnosed an average of 8.8 years after phlebotomy (range 2-18 years) and 324 individually matched controls. Findings indicated an increased risk associated with increasing PFOA exposure (Shearer et al, JNCI, 2020).
To follow up on these findings in a larger and more racially and ethnically diverse population, OEEB investigators conducted a nested case-control study of serum PFAS concentrations and risk of RCC within the Multiethnic Cohort Study (MEC). While PFOA was not associated with RCC risk overall in this racially and ethnically diverse population (Rhee et al, Environment International, 2023), investigators observed suggestive positive associations among non-Hispanic White participants and those with sera collected before 2002; notably, these subgroups are the most comparable to the population of the PLCO study. There was also suggestive evidence of increased RCC risk among those with higher levels of PFNA, another widely detected PFAS; this association was strongest among African American participants. These investigations within the PLCO and MEC are, to our knowledge, the largest studies of PFAS and kidney cancer to date, and the only ones to evaluate associations with directly measured serum PFAS concentrations at ranges of exposure comparable to those seen in the U.S. general population. The findings from the MEC study highlight the importance of evaluating the health effects of PFAS exposures in racially and ethnically diverse populations.
For more information, contact Dr. Jonathan Hofmann.
Shearer JJ*, Callahan CL*, et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma. J Natl Cancer Inst. 2021 *Co-first authors.
Rhee J*, Chang VC*, Cheng I, Calafat AM, Botelho JC, Shearer JJ, Sampson JN, Setiawan VW, Wilkens LR, Silverman DT, Purdue MP*, Hofmann JN*. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma in the Multiethnic Cohort Study. Environ Int. 2023 *Co-first and co-senior authors.
Testicular Cancer
An Investigation of Serum PFAS Levels and Testicular Cancer Risk Within the Department of Defense Serum Repository
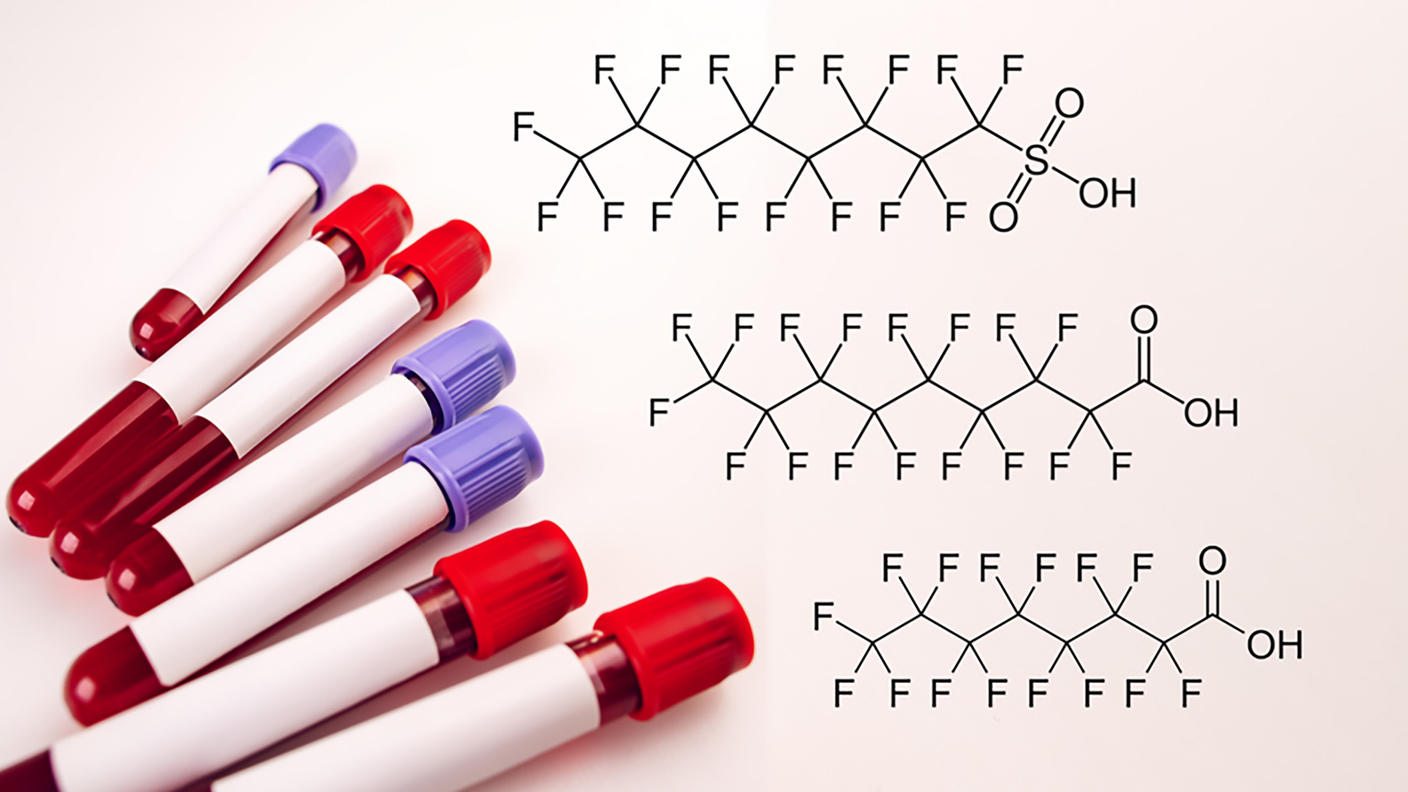
PFAS are a component of firefighting foams used at airports and military installations to extinguish petroleum-based fires. There has been concern over possible health effects from exposures to PFAS, including elevated risks of cancers of the kidney and testis. OEEB investigators, in collaboration with the Uniformed Services University, led a nested case-control study to investigate the relationship between blood levels of PFAS among active-duty Air Force servicemen and testicular cancer. The researchers discovered elevated levels of some PFAS in the blood was associated with serving as a firefighter or at a base with high levels of PFAS in the water supply. In addition, they found that elevated blood levels of perfluorooctanesulfonic acid (PFOS), a specific type of PFAS, was associated with higher risk of developing testicular cancer. These findings motivate continued examination of PFOS exposure and risk of testicular cancer in military and other highly exposed populations.
For more information, contact Dr. Mark Purdue.
Purdue MP, et al. A nested case-control study of serum per- and polyfluoroalkyl substances and testicular germ cell tumors among U.S. Air Force servicemen. Environ Health Perspect. 2023
Breast Cancer
Serum PFOS and PFOA and Risk of Postmenopausal Breast Cancer According to Hormone Receptor Status
It has been postulated that some PFAS could contribute to the development of breast cancer given biologic evidence of their endocrine-disrupting and estrogenic properties; however, the epidemiologic evidence remains limited and inconclusive. Recently, a nested case-control study among French women with 194 postmenopausal breast cancer cases and 194 controls observed a positive association with pre-diagnostic serum PFOS concentrations, particularly for hormone receptor-positive tumor subtypes. To clarify the potential role of PFOS and PFOA in the development of breast cancer, OEEB researchers conducted an investigation using pre-diagnostic measurements of these analytes from a serum metabolomic panel in an existing nested case-control study of postmenopausal breast cancer (621 cases and 621 controls) in the PLCO Prospective Study (Chang et al, IJC, 2023). Results from this study suggested that PFOS and PFOA may be differentially associated with risk of breast cancer subtypes among postmenopausal women, with a positive association between serum PFOS concentrations and hormone receptor-positive tumors, and possibly between PFOA and hormone receptor-negative tumors. Researchers are planning additional efforts to follow up on these findings for PFOS and PFOA and investigate other PFAS in relation to risk of postmenopausal breast cancer overall and by hormone receptor subtype.
For more information, contact Dr. Jonathan Hofmann or Dr. Mark Purdue.
Chang VC*, Rhee J*, Berndt SI, Moore SC, Freedman ND, Jones RR, Silverman DT, Gierach GL, Hofmann JN*, Purdue MP*. Serum perfluorooctane sulfonate and perfluorooctanoate and risk of postmenopausal breast cancer according to hormone receptor status: An analysis in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2023 *Co-first and co-senior authors.
Ovarian and Endometrial Cancer
A Nested Case-Control Study of Serum PFAS Concentrations and Ovarian and Endometrial Cancers in the PLCO Cancer Screening Trial
This is the first study to directly assess personal exposure to PFAS through serologic testing in samples collected prior to diagnosis of ovarian and endometrial cancers. Utilizing a nested case-control design in PLCO, the NCI will evaluate serum samples collected after menopause from 318 ovarian cancer cases, 430 endometrial cancer cases, and a shared control group of 589 cancer-free women.
For more information, contact Dr. Rena Jones.
Prostate Cancer
Nested Case-Control Study of Serum PFAS Concentrations and Risk of Prostate Cancer in the PLCO Cancer Screening Trial
It is unclear from previous epidemiologic studies whether elevated levels of PFOA and other PFAS are associated with prostate cancer incidence or mortality. To clarify this relationship, OEEB scientists investigated the association between serum PFAS concentrations and aggressive prostate cancer risk in a large prospective study (750 cases and 750 controls) within PLCO. Elevated serum PFAS concentrations were not associated with increased cancer risk in this study population. The study findings do not support an association between PFAS exposures and aggressive prostate cancer risk, although associations at higher PFAS exposure levels or with non-aggressive disease cannot be ruled out.
For more information, contact Dr. Mark Purdue or Dr. Sonja Berndt.
Rhee J, et al. A prospective nested case-control study of serum concentrations of per- and polyfluoroalkyl substances and aggressive prostate cancer risk. Environ Res. 2023
Non-Hodgkin Lymphoma and Thyroid Cancer
Serum PFAS Levels and Risks of Non-Hodgkin Lymphoma and Thyroid Cancer
The goal of this study is to investigate whether elevated pre-diagnostic serum concentrations of selected PFAS are associated with increased risks of non-Hodgkin lymphoma (NHL) and thyroid cancer. To address these aims, OEEB is conducting a case-control study within PLCO, including incident pathologically confirmed cases of NHL and thyroid cancer (N = 845 and 188, respectively) and a shared control group (N=979).
For more information, contact Dr. Mark Purdue.
Thyroid Cancer and Childhood Leukemia
PFAS in the Finnish Maternity Cohort: Nested Case-Control Studies of Childhood Leukemia and Thyroid Cancer in Mothers
DCEG is evaluating PFAS exposure and cancer risk in the Finnish Maternity Cohort (FMC) in two nested case-control studies of: (I) prenatal serum levels and acute lymphoblastic leukemia (ALL) risk in children and (II) pre-diagnostic serum levels and papillary thyroid cancer in mothers. Participants are identified by linking FMC participants to the nationwide Finnish Cancer Registry among mothers with available sera. Cases and controls are sampled from primiparous mothers with no history of cancer at the time of first birth, having a full-term (37-42 week) singleton live birth without Down syndrome. The study includes 400 cases of ALL (children <15 years) and 400 cases of thyroid cancer among the mothers, using frequency matching and 400 controls. In the study of thyroid cancer, the researchers detected seven PFAS in more than half of the women. They noted suggestive but imprecise increased risks associated with PFOA and PFOS for those diagnosed with thyroid cancer before age 40, but overall, these results show no clear association between PFAS and papillary thyroid cancer risk.
For more information, contact Dr. Rena Jones.
Madrigal J et al. Prediagnostic serum concentrations of per- and polyfluoroalkyl substances and risk of papillary thyroid cancer in the Finnish Maternity Cohort. Int J Cancer. 2023
Jones RR... Ward MH. et al. Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances and childhood leukemia among offspring in the Finnish Maternity Cohort. J Nat Cancer Inst. 2023
Drinking Water Exposure Assessment in the California Teachers Study (CTS)
The focus of this work is to determine whether a PFAS drinking water exposure assessment is feasible in a California study population and to establish a framework for future studies of PFAS (and other water contaminants). The California Teachers' Study (CTS) is a prospective cohort of 133,479 female members of the California State Teachers Retirement System enrolled in 1995; as of 2015, the number of cancers for key sites of interest included NHL=881; thyroid=455; and kidney=318. Drinking water source information was not previously collected, so NCI developed questions relating to drinking water source, treatment, and intake; frequency of bathing/showering; and duration at the residence for a CTS follow-up questionnaire implemented in 2018-2019. Public drinking water utility boundaries have been collated by the California Department of Public Health—these are passively collected data and lack some historical details, but they provide a potential means to assign people to a public drinking water source based on their residence location. Because no historical PFAS measurements in drinking water are available, OEEB scientists have initiated efforts to evaluate whether serum PFAS levels can be predicted by questionnaire- and GIS-based information about CTS participants, their public water supply, and local environmental characteristics/point sources.
For more information, contact Dr. Rena Jones.