Validation of a Low-cost, Rapid HPV DNA Genotyping Test for Cervical Cancer Prevention
, by Jennifer K. Loukissas, M.P.P.
A key deliverable of the DCEG Cancer Moonshot initiative to Accelerate Cervical Cancer Control is a rapid, mobile, simple, and affordable HPV DNA genotyping assay for risk-based screening in resource-limited settings where routine screening is logistically and cost prohibitive. Kanan T. Desai, M.D., M.P.H., Mark Schiffman, M.D., M.P.H., Silvia de Sanjose, M.D., Ph.D., and colleagues in the Clinical Epidemiology Unit, Clinical Genetics Branch, guided scientists at Atila Biosystems in the redesign of their existing test for this purpose and published their validation study results on June 6, 2022, in the International Journal of Cancer.
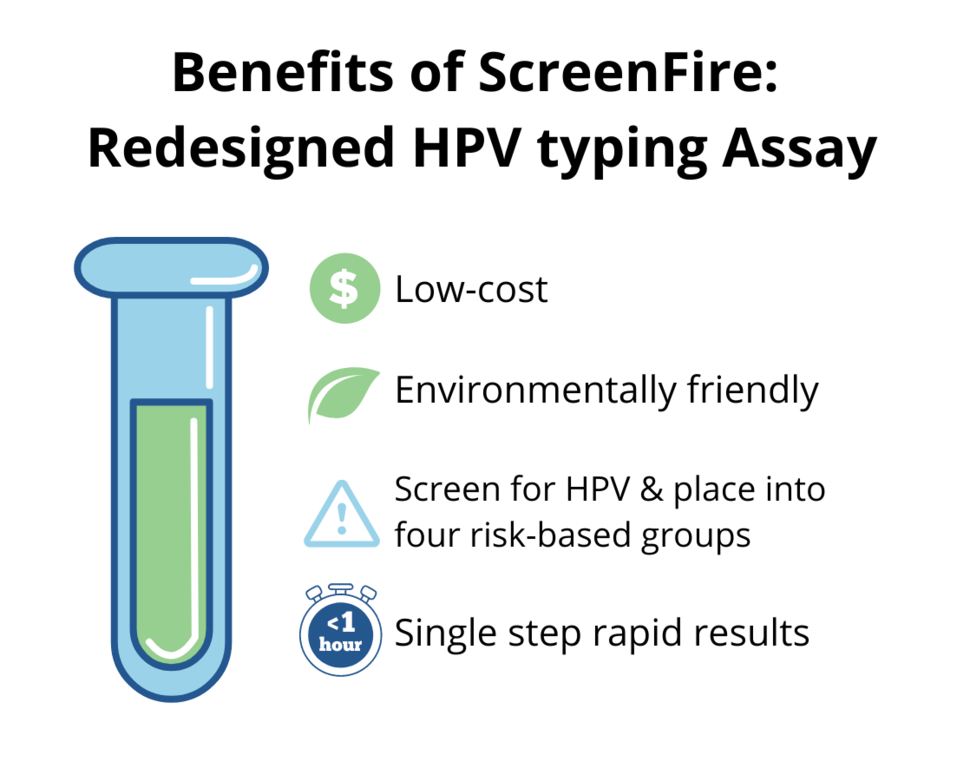
The new mobile platform was evaluated using samples collected from women participating in a population-based screening study in rural Nigeria. The investigators reported very good agreement between the laboratory standard (PCR) and the redesigned test. A key feature of the new test is that it provides, at minimal additional cost, hierarchical results by HPV type for risk stratification, in order of clinical importance of the four type groups of greatest concern for cervical precancer and cancer risk. Those groups are (1) HPV 16; (2) HPV 18/45; (3) HPV 31/33/35/52/58; and (4) HPV 39/51/56/59/68. Results are generated within 20 to 60 minutes, without the need for DNA extraction.
The authors emphasize the need for this assay noting that while HPV vaccination will provide the best possible prevention for younger women, “older unvaccinated birth cohorts will remain at risk, making secondary prevention efforts essential over the coming decades.”
Follow-up studies are underway to evaluate performance of the test on self-collected samples compared to histopathologic outcomes.
Reference
Desai KT, et al. Redesign of a rapid, low-cost HPV typing assay to support risk-based cervical screening and management. Int J Cancer. 2022.