Primary Prevention of Cervical Cancer Through Human Papillomavirus (HPV) Vaccination: The HPV Vaccine Trials
The HPV vaccines, co-invented by NCI researchers, have been proven safe and effective and have been in use for nearly two decades. Despite availability, uptake in the United States and elsewhere was unacceptably low. DCEG and Costa Rican researchers discovered that one dose might provide adequate protection through the long-term follow-up of HPV-vaccinated women aged 18–25 years in the original Costa Rica HPV Vaccine Trial (CVT).
Wide-spread adoption of a single-dose HPV vaccine schedule would facilitate dramatic increases in global uptake. New randomized studies were needed to provide actionable evidence required to change vaccine policy.
NCI and Costa Rica investigators launched the ESCUDDO efficacy trial (“Estudio de Comparacion de Una y Dos Dosis de Vacunas Contra el Virus de Papiloma Humano (VPH)”). ESCUDDO was supported in part by the Cancer Moonshot.
ESCUDDO succeeded in showing that one dose of HPV vaccine works as well as two doses to prevent infections with the types of HPV that cause most cervical cancers. After five years of follow-up, we found that a single dose of either a bivalent or nonavalent HPV vaccine provided similar protection to that of two doses. The vaccine effectiveness against HPV16 or HPV18 was at least 97% in each of the four trial groups.
ESCUDDO was complemented by multiple other trials, two of which are also conducted by NCI and Costa Rica investigators: the PRIMAVERA Immunobridging Trial and the PRISMA Efficacy Trial.
Research continues in the CVT to document durability of single- and multiple-dose HPV vaccine regimens. This unique opportunity is possible thanks to the collaboration with Costa Rica, ACIB-FUNIN (acibfunin.com), and continued support of the NCI Intramural Research Program.
For more information, contact Aimée R. Kreimer, Ph.D.
Infections and Immunoepidemiology Branch - Research Areas
Secondary Prevention through Screening and Management for Accelerated Cervical Cancer Control
There are several challenges to cervical cancer control worldwide that differ in high- and low-resource settings. Areas with ample resources face issues of inefficient screening, overtreatment, and low HPV vaccine uptake in some places. In contrast, areas with limited resources struggle with lack of access to screening tests, unsustainable multi-visit screening programs, limited treatment options, and low access to HPV vaccines.
To overcome these barriers, NCI researchers are addressing four major areas for accelerated cervical cancer control:
- Advance understanding of HPV and multi-stage carcinogenesis. Building upon decades of research to understand HPV and multi-stage carcinogenesis, NCI researcher conduct studies to (1) estimate transition probabilities between HPV infection, precancer, and cancer, and (2) better characterize squamous and glandular precancers and cancers.
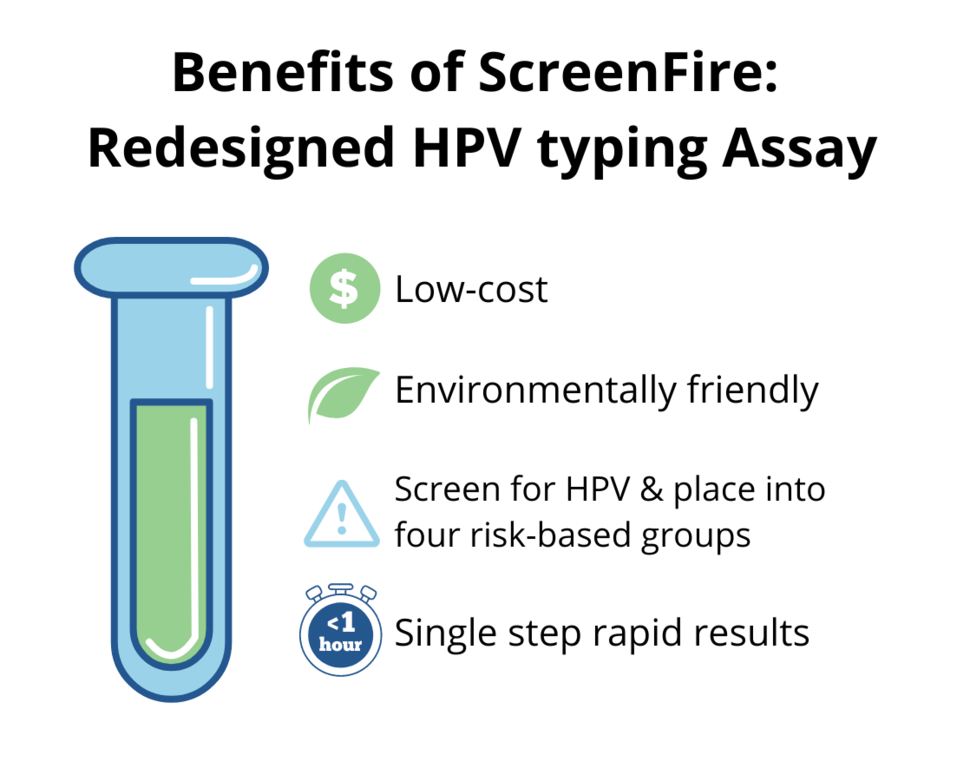
- Invent and validate novel assays and prevention methods. NCI researchers are developing new technologies that are being validated in large diverse clinical populations, including HPV genotyping assays for studies of natural history, vaccination, and screening/management and low-cost, rapid HPV DNA tests for screening in low-resource settings. New assays like HPV methylation, automated dual stain, and automated visual evaluation based on artificial intelligence are being evaluated for triage of HPV-positive individuals in a worldwide network of studies. Vaginal self-collection is evaluated to expand the reach of screening and triage, particularly in low-resource settings.
- Unify national and international guidelines and recommendations. Risk prediction models are being developed for uniform management of abnormal screening and triage results and an infrastructure was created to develop and review new evidence from population-based and other studies to continually improve clinical management. Using these methods and technologies, NCI researchers have developed approaches that allow estimating the risk of cervical precancer and cancer with high accuracy and are providing solutions for screening, triage, management, and treatment for a wide range of settings. The Federal Cervical Cancer Collaborative was established to develop resources for sustained efforts to accelerate cervical cancer control in safety-net settings of care in the U.S. and territories.
- Enable global control efforts through integration of screening and vaccination. Successful completion of the Moonshot goals on cervical screening, management, and treatment will yield novel single-visit screen-and-treat strategies. Paired with 1-Dose HPV vaccination, these programs will allow to the establishment of integrated HPV screening and vaccination campaigns in low-resource settings.
For more information, contact Mark Schiffman and Nicolas Wentzensen.
Clinical Epidemiology Unit - Research Areas