Somatic Mutations in Routinely Collected Cervical Cells Associated with Infection Outcome and HPV Type
, by Jennifer K. Loukissas, M.P.P.
Cells collected from the cervix during routine screening for cervical cancer can reveal the accumulation of somatic mutations that are associated with precancer and cancer, demonstrating their potential as a biomarker of carcinogenesis in individuals who test positive for infection with human papillomavirus (HPV). The findings were published in Nature Communications on September 12, 2024.
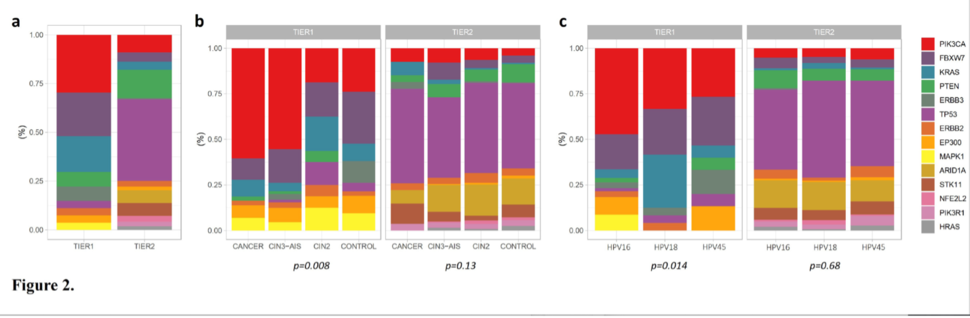
Investigators in the Clinical Genetics Branch, led by Senior Investigator Lisa Mirabello, Ph.D., and former research fellow Maísa Pinheiro, Ph.D., used deep targeted sequencing to look for hotspot driver mutations resulting from persistent infection with one of the three most carcinogenic types of HPV (HPV 16, 18, and 45). They found that these mutations were significantly more prevalent in HPV-positive individuals who went on to develop precancers and cancers (up to 76 times more common in cancers), depending on mutation type and HPV type, compared with HPV-positive individuals who did not develop precancer or cancer. The approach was particularly useful in identifying glandular precancer lesions, which can develop into adenocarcinoma. This cancer is more difficult to detect with cytology alone. Importantly, the mutations were present up to six years before clinical cancer diagnosis.
A specific subset of hotspot mutations was rare in the controls (2.6%) but significantly more prevalent in precancers, particularly glandular precancer lesions (10.2%), and cancers (25.7%), supporting their involvement in carcinogenesis. The analysis was conducted on 3,929 HPV-positive exfoliated cervical cells collected during routine screening in the Kaiser Permanente Northern California (KPNC)-NCI HPV Persistence and Progression Cohort. All samples were collected prospectively. In a subset of 396 women, the investigators had serial samples collected prior to diagnosis, which allowed them to evaluate mutation changes over time.
Nearly all cervical cancers are caused by persistent infection with carcinogenic HPV. In addition to prophylactic vaccination against the most carcinogenic types, routine screening and clinical management of abnormal results can drastically reduce incidence of cervical cancer by removing precancerous changes.
The distribution of observed hotspot mutations differed by HPV type and within type lineages/sublineages. HPV 18/45-positive cervical cancers were more likely to have multiple hotspot mutations compared to HPV 16-positive. The proportion of cells containing hotspot mutations (i.e., higher variant allele fraction) increased from controls through precancers and cancer cases, suggesting clonal expansion in cancer development.
While the authors note that their findings should be confirmed, the proof-of-concept has the potential to be translated into clinical practice. Hotspot mutation detection is less expensive and faster than full molecular profiling. Additionally, the approach uses exfoliated cells collected for routine screening, which adds the important element of convenience.
Reference
Pinheiro M et al. Somatic mutations in 3929 HPV positive cervical cells associated with infection outcome and HPV type. Nat Comms. 2024.