Overview
This population-based case-control study was conducted from 2000-2003 in two major cities in Poland, Warsaw and Lodz, and enrolled 2,386 breast cancer cases and 2,798 age and site matched controls.
Study Team
Mustapha Abubakar, M.D., Ph.D., Earl Stadtman invetigator, Integrative Tumor Epidemiology Branch (ITEB)
Jonine Figueroa, Ph.D., M.P.H., senior investigator, ITEB
Background & Study Design
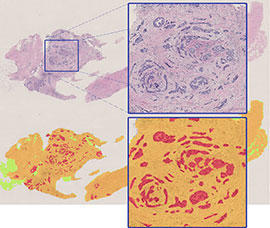
The Polish Breast Cancer Case-Control Study utilized state-of-the art exposure assessment techniques, including detailed personal interviews, anthropometric measurements, physical activity monitors, and collection of dust samples from the participants’ homes. In order to facilitate study of a wide range of biomarkers, biological specimen collection included blood samples processed as cryopreserved whole blood, serum+ blood clot, plasma+buffy coat+red blood cells; 12-hour overnight urine; paraffin-embedded tumor and normal tissue; and fresh tissue from tumors, non-neoplastic breast tissue, and mammary fat tissue.
Investigators obtained ten-year follow-up information from medical records for approximately 1,300 breast cancer cases recruited in Warsaw and 300 cases recruited in Lodz, as well as mortality data on most cases in the study using the Cancer Registry and Death Certificate Office database. The main research projects included:
- Analyses of questionnaire-based factors and anthropometric/physical activity measurements in relation to cancer risk and clinical outcomes;
- Identifying genetic susceptibility markers of cancer risk and clinical outcomes using candidate gene and genome-wide association (GWAS) approaches;
- Analyses of breast tissue/tumor markers to evaluate relationships with cancer risk factors (known or suspected), and their impact on predicting recurrence and survival after diagnosis;
- Analyses of biomarkers in DNA (somatic changes), cryopreserved blood cells, serum/plasma, and urine in relationship to cancer risk and clinical outcomes