Overview
The National Cancer Institute (NCI) conducted the Transplant Cancer Match Study to examine cancer risk in solid organ transplant recipients. The study was a close collaboration among researchers and staff at various federal and state government agencies:
- The Health Resources and Services Administration (HRSA), which oversees solid organ transplantation in the U.S.
- The National Cancer Institute
- The Scientific Registry of Transplant Recipients (SRTR)
- Multiple state and regional cancer registries throughout the U.S.
The study did not recruit participants, because it used only data previously collected by public health agencies.
Study Team
Principal investigator: Eric Engels, M.D., M.P.H.
Study coordinator: Kelly J. Yu, Ph.D., M.P.H.
Collaborators
Background and Purpose
Solid organ transplantation provides life-saving therapy for patients with end-stage organ disease. However, because solid organ transplant recipients receive long-term immunosuppressant medications to prevent organ rejection, they have an elevated risk of cancer that is two to four times higher overall than the risk seen in the general population.
Much of the increased risk is related to a high risk for cancers caused by viral infections. The most common of these virus-related cancers is non-Hodgkin lymphoma, which is frequently caused by Epstein-Barr virus. Other virus-related cancers include Kaposi sarcoma (caused by human herpesvirus 8); cancers of the cervix, vagina, vulva, penis, anus, and oral cavity (caused by human papillomavirus); and liver cancer (caused by hepatitis C and B viruses). Certain other malignancies, such as cancers of the lung, kidney, and thyroid, are also increased in transplant recipients. In addition, skin cancers (especially squamous cell and basal cell carcinomas, but also melanoma) occur at an elevated frequency.
For public health purposes, both organ transplantation and cancer are monitored by U.S. federal and state agencies. Information on all U.S. transplant candidates, recipients, and donors is provided by transplant centers and organ procurement organizations to the Scientific Registry of Transplant Recipients (SRTR). Cancers are reportable by clinicians and healthcare facilities to state and regional cancer registries.
A major goal of the Transplant Cancer Match Study was to determine the overall pattern of cancer in transplant recipients and identify key risk factors for individual cancer types. These findings have yielded information on the role of the immune system in the development of cancer. In addition, a better understanding of cancer risk factors has helped to clarify the contributions of medical conditions, chronic viral infections, and specific medications.
While cancer risk is elevated in transplant recipients, the benefits of organ transplantation for people with end-stage organ disease far outweigh this risk. Nonetheless, HRSA and the SRTR continuously monitor transplant outcomes to maximize the safety of solid organ transplantation. Because cancer is an important adverse outcome of transplantation, findings from the Transplant Cancer Match Study can help identify opportunities to prevent or screen for cancer and optimize survival following a cancer diagnosis.
Study Design
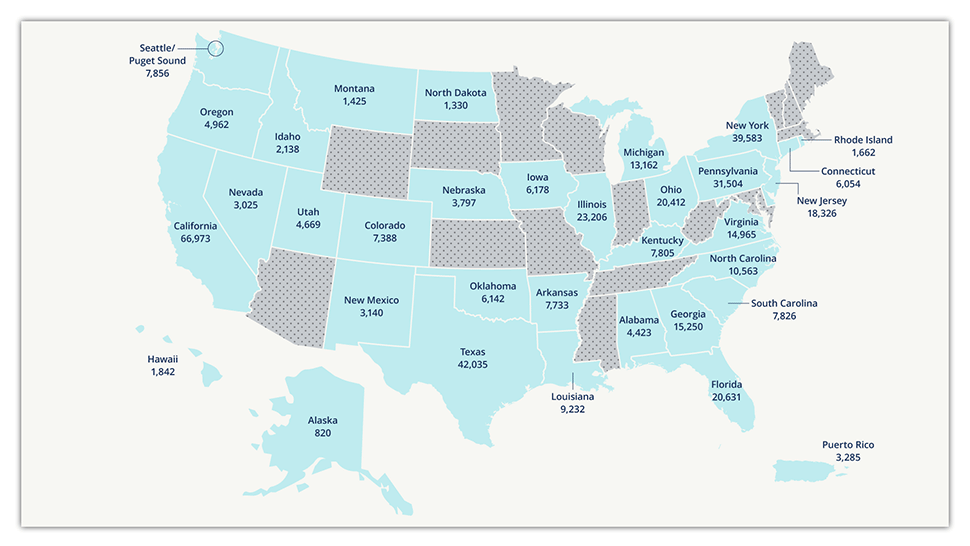
The Transplant Cancer Match Study uses electronically linked data from the SRTR and cancer registries to study the epidemiology of cancer in the U.S. transplant population. This study is the largest study of cancer risk in solid organ transplant recipients in the world. As of April 2022, the study includes cancer data on 68% of the U.S. transplant population from 1987 through 2019, or approximately 511,000 transplant recipients. The study captured recipients of all organ types in each participating state or metropolitan region, so the included population is representative of the overall U.S. transplant population. Ongoing registry linkages provide updated data to monitor cancer trends and to expand the study into new geographic regions of the U.S. Find more information in the Transplant Cancer Match Study: Methods Description.
Study Results and Select Publications
Using data from the Transplant Cancer Match Study, DCEG researchers have characterized the spectrum of cancer risk in transplant recipients, identified risk factors for cancer in this population, and evaluated survival outcomes following a cancer diagnosis.
For example, an analysis of diffuse large B-cell lymphoma, a common and highly aggressive form of NHL, revealed an almost 14-fold elevation in the risk of this NHL subtype among transplant recipients compared with the general population.
These findings and others have helped clarify the role of immunosuppression in shaping cancer incidence and survival, and have been impactful in shaping the clinical management of transplant recipients.
Selected publications from this research include the following:
- Spectrum of Nonkeratinocyte Skin Cancer Risk Among Solid Organ Transplant Recipients in the US
- Predicted cure and survival among transplant recipients with a previous cancer diagnosis.
- Risk of rare cancers among solid organ transplant recipients.
- Survival after a cancer diagnosis among solid organ transplant recipients in the United States.
- Increased risk of lymphoma following organ transplantation in childhood.
- Increased risk of colorectal cancer in lung transplant recipients with cystic fibrosis.
- Variation in cancer incidence among patients with end-stage renal disease during kidney function and nonfunction intervals.
- Spectrum of cancer risk among solid organ transplant recipients, including non-Hodgkin lymphoma and 31 other individual types of cancer.
See all publications from the Transplant Cancer Match Study.
Collaboration and Data Sharing
Ideas for specific projects and collaborations related to these aims are welcome. The Transplant Cancer Match Study principal investigator is the custodian for the data. Agreements between the National Cancer Institute, the Health Resources and Services Administration, and participating registries do not allow the investigators to share the data with outside researchers. Also, the study data are complex and require the use of sophisticated data handling and statistical modeling techniques. Collaborators can use the study data at the National Cancer Institute under close supervision by the principal investigator, or can participate in analyses through review of tabulated data.
For more information, contact:
Eric A. Engels, M.D., M.P.H., Principal Investigator
Infections & Immunoepidemiology Branch
Division of Cancer Epidemiology and Genetics
National Cancer Institute
9609 Medical Center Drive, Room 6E102