International Agency for Research on Cancer Produces New Cervical Cancer Screening Handbook
, by Jennifer K. Loukissas, M.P.P.
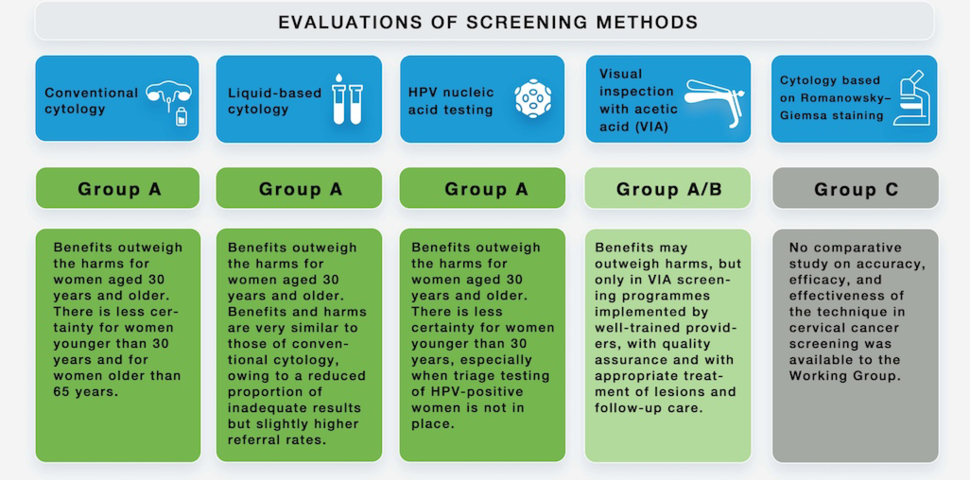
DCEG senior investigators Nicolas Wentzensen, M.D., Ph.D., M.S., and Mark Schiffman, M.D., M.P.H., have led numerous efforts to improve cervical cancer prevention, screening, and management guidelines. Most recently, Dr. Wentzensen co-chaired and Dr. Schiffman participated in a working group convened by the International Agency for Research on Cancer (IARC), to produce the IARC Handbook of Cancer Prevention for cervical cancer. In 2020, the group conducted an extensive evidence review summarizing the published literature on cervical screening and wrote the Handbook, which will be published in 2022. Key results from the evidence review were summarized in a special report published November 10, 2021, in The New England Journal of Medicine.
Major findings from the evidence report include that HPV DNA testing for cervical screening has higher effectiveness and better balance of benefits and harms compared to other approaches. These findings support recently updated cervical screening guidelines that emphasize the role of HPV testing in primary screening. Data supporting these conclusions came—in part—from research conducted by the Clinical Epidemiology Unit. The IARC Handbooks of Cancer Prevention provide definitive evaluations about which measures can prevent or detect cancer at an early stage. Evaluations are conducted by a group of interdisciplinary experts who collect all the relevant studies published to date, review the data, and present their expert conclusions and recommendations on how to reduce risk of cancer.
Read the article and press materials on the IARC Website.
Reference
Bouvard V et al, The IARC perspective on cervical cancer screening. N Engl J Med. November 2021.