Enduring Guidelines: Methods and Dual Stain for Cervical Cancer Screening Triage
, by Jennifer K. Loukissas, M.P.P.
Cervical cancer is preventable through vaccination against human papillomavirus, screening, and clinical management of abnormal results. Following the establishment of the 2019 ASCCP Consensus Guidelines for Clinical Management—the first risk-based guidelines for cervical cancer—the Enduring Guidelines effort was launched to ensure timely update of the guidelines with rapidly emerging technologies that receive regulatory approval and new scientific evidence.
A new paper (Wentzensen et al.) describes the Enduring Guidelines approach and methods. A second paper (Clarke et al.) presents the first clinical recommendations from the Enduring Guidelines group on the use of dual stain cytology to manage abnormal cervical screening results. Both were published March 6, 2024, in the Journal of Lower Genital Tract Diseases.
In the first paper, Nicolas Wentzensen, M.D., Ph.D., M.S., senior investigator in the Clinical Genetics Branch (CGB) and head of the Clinical Epidemiology Unit, and colleagues, describe the principles and methods of the Enduring Guidelines effort: to recommend new technologies and approaches that are as parsimonious as possible to minimize the number of tests and clinical evaluations while maximizing detection of precancer and reassurance against developing cancer. They also describe a new approach developed by the technology assessment group to estimate resource utilization of different approved strategies in a screening setting. These estimates can support the introduction of different strategies in healthcare settings. The Enduring Guidelines group is working closely with developers of clinical decision tools to implement new recommendations to support providers and patients.
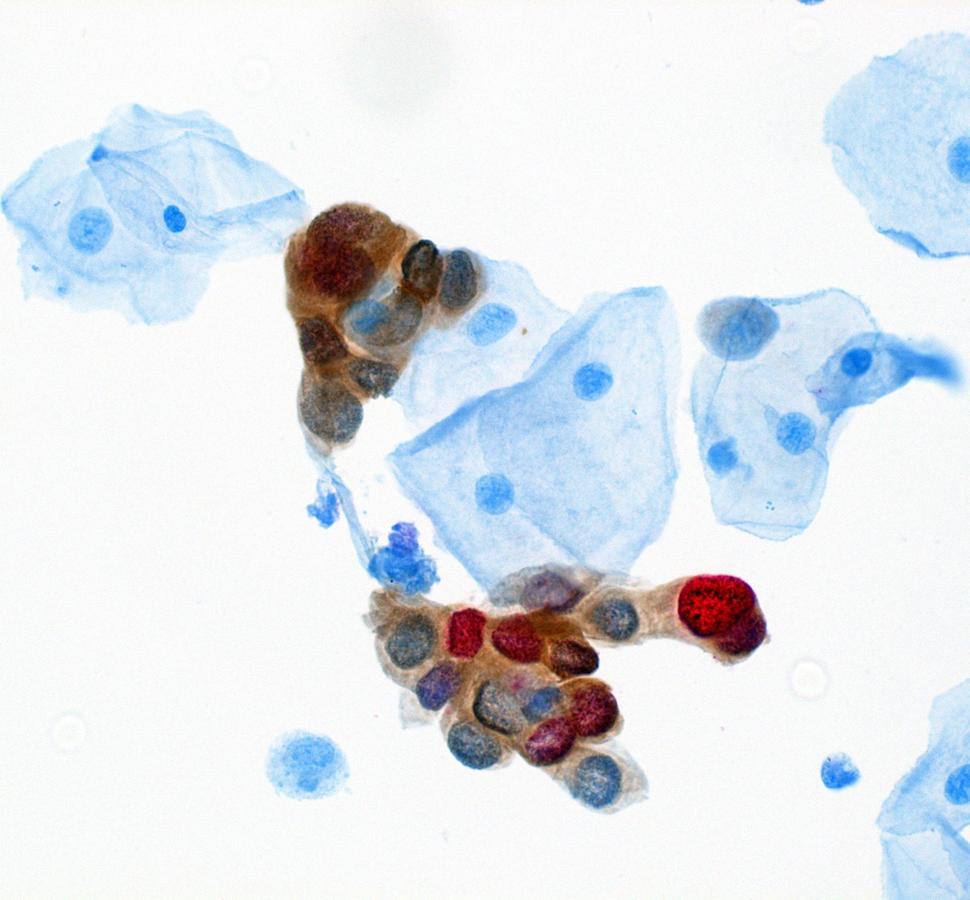
The second paper presents recommendations and data to support the inclusion of dual stain in management of abnormal screening results. Risk estimates supporting these recommendations came from two DCEG-led studies, the IRIS cohort out of Kaiser Permanente Northern California and the STRIDES cohort out of the University of Mississippi and Mississippi State Department of Health. Megan Clarke, Ph.D., M.H.S., Earl Stadtman investigator in CGB, and colleagues, describe the improved risk stratification provided by dual stain, compared to cytology, and the simplified management approaches it offers with better identification of patients requiring colposcopy and better reassurance for patients with a normal dual stain result. Importantly, dual stain risk estimates were found to be portable across different populations. The results were very similar in both cohorts. As a result, recommendations for dual stain are being integrated into existing clinical decision support tools to facilitate their implementation and adoption.
References
1. Wentzensen N et al. Enduring Consensus Guidelines for Cervical Cancer Screening and Management: Introduction to the Scope and Process. J Low Genit Tract Dis. 2024.
2. Clarke MA et al. Recommendations for Use of p16/Ki67 Dual Stain for Management of Individuals Testing Positive for Human Papillomavirus. J Low Genit Tract Dis. 2024.