Elizabeth Cahoon and Environmental Radiation Research: Investigator Profile
, by Elise Tookmanian, Ph.D.
Elizabeth K. Cahoon, Ph.D., M.S., Earl Stadtman investigator in the Radiation Epidemiology Branch, helms an innovative research program that uses the power of epidemiology to discover how environmental radiation affects human health by developing new resources for quantifying exposures and identifying populations that may be especially vulnerable.
“Epidemiology is such a powerful tool for seeking out truth about human health,” Elizabeth K. Cahoon, Ph.D., M.S., Earl Stadtman investigator in the Radiation Epidemiology Branch (REB) said. “We can learn so much from carefully designing a study and implementing the powerful statistical tools we have available.” Ultimately, cancer epidemiological studies can inform screening and prevention approaches to decrease the burden of disease on our society.
Like many epidemiologists, Dr. Cahoon’s path to the field was serendipitous. She earned a bachelor’s degree in bioengineering from the University of Pennsylvania, Philadelphia, and then joined a consulting firm for a few years. “But of course,” she said, “at 22 years old, what can you really consult about? You don’t know much yet.” She wanted to build expertise, so pursued a master’s degree in systems engineering with a health policy focus at the Massachusetts Institute of Technology, Cambridge. During the course of her studies, she took an epidemiology class, and something clicked. “Here was an entire field of research dedicated to solving puzzles about disease causation on a population level, with enormous potential for reducing health inequalities through prevention,” said Dr. Cahoon. Following her master’s program, she began a doctorate focusing on environmental epidemiology at Johns Hopkins University, Bloomberg School of Public Health, Baltimore, Maryland. Environmental epidemiology focuses on the health effects of factors that are often outside of an individual’s immediate control and may impact a substantial part of the population.
“By identifying and quantifying risks associated with these exposures, there is an opportunity to reduce health inequalities by influencing guidelines and informing decision-making,” said Dr. Cahoon. “It also really appealed to me that epidemiology is observational; it doesn’t necessarily interfere with the course of a person’s life. But for that same reason, many factors may influence the results.” Epidemiologists aim to define and minimize these limitations by strengthening their data sources and analytical methods. “Did we collect the data in the best way possible? Are we analyzing it appropriately? The things that are in our control are very much in our control.” Her current research, described below, embodies these principles, from developing new resources to quantify exposures to questioning long-held assumptions.
Environmental Radiation Epidemiology
To continue her training, Dr. Cahoon joined REB as a postdoctoral fellow. The multidisciplinary approach of the Division, with experts in epidemiology, medicine, dosimetry, health physics, and biostatistics, appealed to her, as well as the ability to focus on environmental radiation and human health in the context of long-term international collaborations. Her research includes studies of ultraviolet (UV) radiation in sunlight and environmental sources of ionizing radiation.
“I like to think about how our day-to-day experience in the world has changed over time,” said Dr. Cahoon. Global migration has influenced where people live and the types of exposures in their environment. For example, individuals with ancestors from northern Europe now live in parts of the U.S. and Australia where ambient UV radiation is much higher and consequently, they develop skin cancer at much higher rates. As most people spend far more time indoors today compared to 100 years ago, contemporary patterns of exposure to UV radiation may have different implications for cancer risk.
Discovering nuclear fission, and the ability to carry it out at scale, has also changed our environment. While we are all exposed to small levels of natural background radiation, nuclear technologies have the potential to drastically change exposures for certain populations. Dr. Cahoon leads studies on the health effects among individuals exposed to radiation from the Chernobyl nuclear power plant accident and nuclear bomb detonations.
Ultraviolet Radiation
UV radiation is the primary risk factor for skin cancer, a relationship that has been established for many years. However, Dr. Cahoon says, “there is still a lot to discover.” As an avid gardener, she spends much of her free time outdoors and wants others to enjoy being outdoors, socializing or being active, safely.
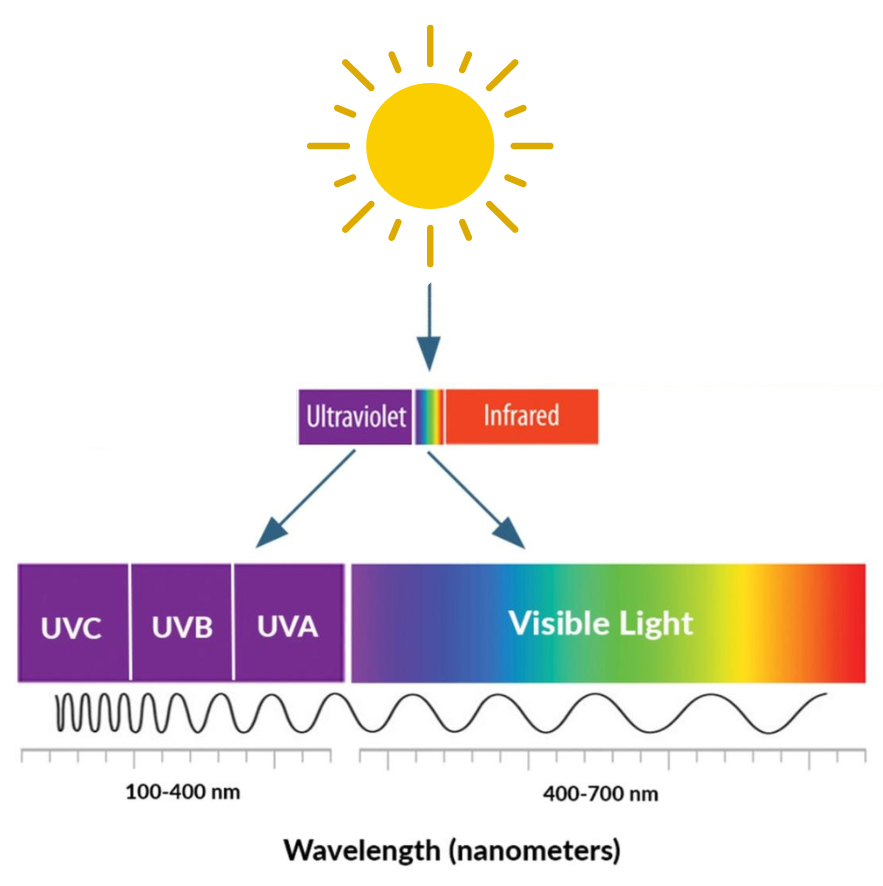
Ninety-five percent of UV radiation that reaches the earth’s surface is the longer wavelength, lowest energy UV radiation (UVA). The remaining five percent is shorter wavelength, higher energy UV radiation (UVB) that can damage DNA directly and has been shown to cause skin cancer. For this reason, policies informing the production of sunscreen, window glass, and the type of light emitted by tanning beds have focused on reducing exposure to UVB in order to prevent skin cancer. These policies have engineered our environment to have a higher proportion of UVA than present in natural sunlight. UVA penetrates deeper into the skin and has been shown to cause DNA damage indirectly. To best inform skin cancer prevention efforts, Dr. Cahoon is investigating the risks associated with relative exposure to different wavelengths of UV radiation. “If UVA is found to be strongly associated with skin cancer risk, there could be implications for sunscreen formulations and window glass,” she said. She is examining this question in large nationwide cohorts such as the U.S. Radiologic Technologists (USRT) study.
Another major area of research for Dr. Cahoon is the effect of certain medications on skin cancer risk. Common photosensitizing agents include exogenous and endogenous hormones and some common medications (e.g., nonsteroidal anti-inflammatory drugs (NSAIDs), thiazide diuretics, antifungals, antihistamines). Specifically, she is interested in whether individuals taking these medications are more vulnerable to the effects of UV radiation. In the USRT cohort, she found significantly increased risks of basal cell carcinoma, a common form of skin cancer, with use of several photosensitizing agents, including prescription diuretics and menopausal hormone therapy but not NSAIDs. No significant interactions were found with ambient UV radiation, eye color, hair color, or skin complexion.
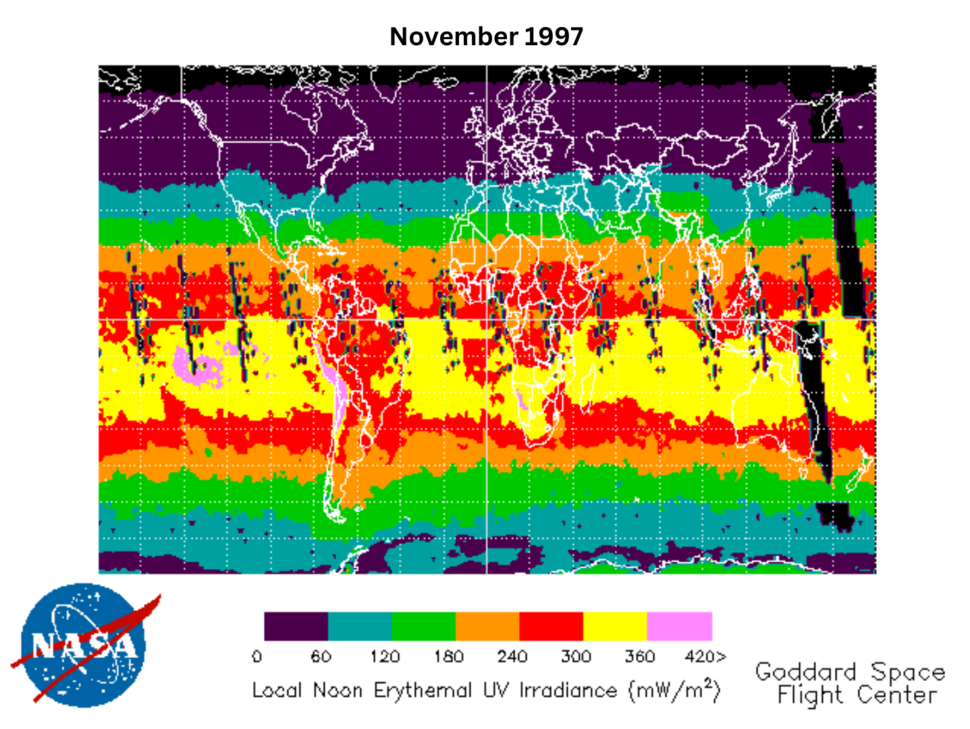
For some rare cancers, there has been suspicion of an association with greater exposure to UV, but the small number of cases in any one study makes this hypothesis difficult to evaluate. To collect enough data to address this question and estimate long-term UV radiation in different populations, Dr. Cahoon created a database that links residential locations to satellite-based daily noontime ambient UV radiation estimates, which was developed by the National Aeronautics and Space Administration. With this database, Dr. Cahoon can link to various datasets, like the USRT cohort or the Surveillance, Epidemiology, and End Results Program (SEER) cancer registry, and examine the relationship between UV exposure and rare cancers, including cutaneous angiosarcoma, sebaceous carcinoma, ocular melanoma, Kaposi Sarcoma, and salivary gland cancer.
Interestingly, salivary gland cancer occurs at much higher rates in patients with a skin cancer diagnosis. This suggests that they may share a common risk factor, such as UV exposure. To investigate, Dr. Cahoon and Jim Mai, M.D., M.P.H., Ph.D., REB research fellow, linked the location-based UV radiation estimates to data from SEER. When they looked at salivary gland cancer by histological subtype, they discovered that only the squamous cell carcinoma subtype in non-Hispanic White individuals was associated with greater UV exposure. Clinical reports have indicated that distinguishing between primary squamous cell carcinoma of the salivary gland and skin cancer metastases using histology alone is difficult, suggesting squamous cell carcinomas of the salivary gland in people with skin cancer may in fact be metastases. Confirming these results could lead to new salivary gland cancer screening procedures for this high-risk group.
While her research continues to reveal new information about UV radiation and cancer risk, Dr. Cahoon has one piece of advice for enjoying the sun: “Wear a hat. Eighty-to-ninety percent of the most common skin cancers occur on the head and neck. If everyone wore a hat, a lot of skin cancer cases could be prevented.”
Ionizing Radiation
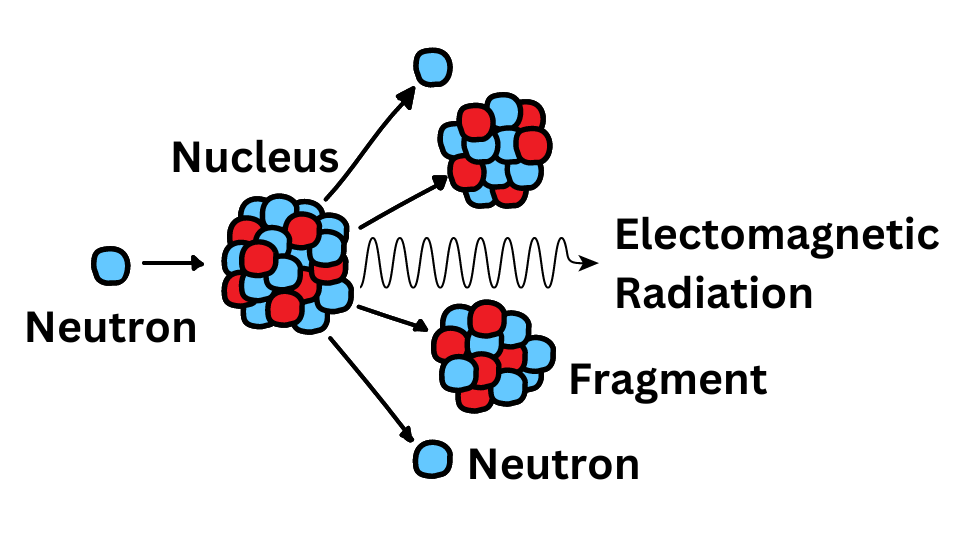
Ionizing radiation is more harmful to human health than UV radiation because it is higher in energy. This means it can break chemical bonds more easily, including those that maintain DNA. The ionizing radiation released following nuclear accidents is often accompanied by radioactive isotopes, including iodine, which can continue to emit radiation as they decay.
REB has been studying populations exposed occupationally and residentially to fallout from the Chernobyl power plant accident, alongside partners in Belarus, Ukraine, and Russia, since the late 1990s. The intramural research program at the NCI provides a stable environment for long-term, international, multidisciplinary studies of these exposures. As Dr. Cahoon was promoted to tenure track, she has taken on more leadership in this area. In 2019, she was appointed as the principal investigator of DCEG Research on Chernobyl. In her own research portfolio, she focuses on identifying new potentially susceptible populations and quantifying cancer risks using high-quality radiation dosimetry.
Dr. Cahoon is investigating the radiation dose-response for diseases of the thyroid gland among populations exposed to fallout. Because the thyroid uses iodine to synthesize hormones, one of the primary health effects found after the accident was a high dose-response between radioactive iodine and thyroid cancer. But few studies have evaluated the relationship between radioactive iodine exposure and thyroid nodules, a potential precursor to thyroid cancer. This research has the potential to reveal new insights into thyroid cancer etiology by looking into different stages of carcinogenesis and offering opportunities to address precursor lesions before they develop into cancer. “These will be the first studies to evaluate whether nodule characteristics associated with increased risk of thyroid cancer, such as size and vascularization, are apparent at nodule diagnosis or whether these characteristics emerge and evolve over time,” said Dr. Cahoon. She is currently reexamining incidence of new thyroid nodules in Belarus and Ukraine and the progression of prevalent nodules.
Recently, Dr. Cahoon investigated breast cancer risk in women who were nursing at the time of the accident. It is known that radioactive iodine from fallout exposure can concentrate in breast milk. There is concern that the resulting exposure to the surrounding tissue can cause breast cancer. However, even 35 years later, breast cancer risk among those exposed women had not been assessed. “For a long time, we heard that women were told to immediately stop breastfeeding after the accident in order to reduce their radioiodine exposure and associated risk. Anyone who has nursed a baby knows that’s not practical,” said Dr. Cahoon. Obstacles range from lack of access to formula or uncontaminated water and risk of mastitis, a painful infection in the breast that can occur when a mother stops breastfeeding abruptly.
When she explored this question, Dr. Cahoon discovered a 2.5-fold increased risk of breast cancer for women who were breastfeeding at the time of the accident, compared to women in the general population. This finding demonstrates the need for public health guidance that is practical and addresses barriers to implementation. “It’s important to question long-held assumptions when you have good reason to, such as, in this case, whether women were able to stop breastfeeding immediately after the accident.”
Building Relationships
Dr. Cahoon pursues and cultivates strong collaborative partnerships with researchers in the U.S. and abroad. In her role as the principal investigator of DCEG Research on Chernobyl, she has prioritized developing relationships with long-standing collaborators in Ukraine and Belarus. She is also a naturalized U.S. citizen born in the former Soviet Union, and her fluency in Russian language and culture helped to foster these relationships. She also established ongoing collaborations with epidemiologists and data managers from Ukraine and Belarus for support on studies of cleanup workers and exposed children.
Her focus on forming strong relationships is echoed in her mentoring style. “I really love mentoring, which is one of the reasons I came to the intramural program,” she said. During the five years she spent studying for her Ph.D., she tutored dozens of students in biostatistics and epidemiology. She met some at their most vulnerable moments, and through understanding and patience, worked with them to restore their confidence and meet the challenge of their courses. In her mentees, she hopes to instill the love of epidemiology that she discovered in graduate school: “When somebody comes through the door, even if they have very little epidemiology background, I love to get them excited about what they can discover.”