Making Strides Toward Precision Medicine for Individuals with Li-Fraumeni Syndrome
, by Maura Kate Costello, M.A.
Researchers in the Clinical Genetics Branch reveal nuanced cancer incidence patterns within the LFS population and identify areas for improvement in TP53 variant classification and clinical management.
Dramatically Increased Risk of Cancer
Li-Fraumeni Syndrome (LFS) is a rare disorder that predisposes individuals to multiple, early-onset cancers throughout their lives, and it is caused by pathogenic, or likely pathogenic (P/LP) germline variants in the tumor protein 53 gene (TP53). When working properly, this gene is responsible for DNA repair, earning it the title “guardian of the genome.” However, when TP53 is altered—either at the germline or somatically in tumor tissue, the loss of function has profound effects on cancer risk and progression.
One of the key characteristics of LFS is the age at which cancers arise in affected individuals: approximately half of first LFS-associated malignancies occur in women before their early 30s and in men before their mid-40s. In a 2021 study of 480 TP53 P/LP-variant carriers enrolled in the National Cancer Institute’s (NCI)-LFS study (NCT01443468), Kelvin C. de Andrade, Ph.D., M.Sc., postdoctoral fellow in the Clinical Genetics Branch (CGB), Payal Khincha, M.B.B.S., M.S.H.S., assistant clinical investigator in CGB and principal investigator of the NCI-LFS study, and colleagues, further quantified cancer incidence in the LFS population—both overall and broken down by age—in order to inform personalized clinical management and tailored screening.
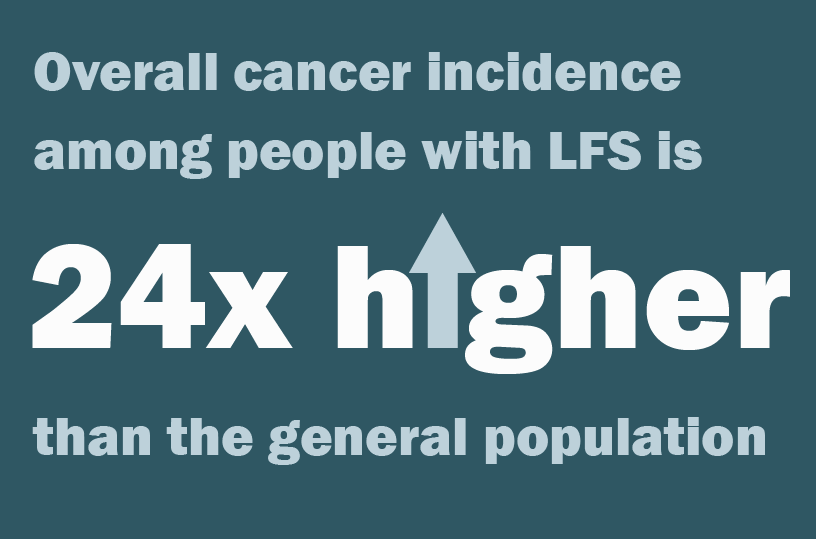
“Our results were staggering. Overall cancer incidence among people with LFS is 24 times higher than the general population,” explained Dr. de Andrade. Investigators observed the highest cancer incidence among individuals with LFS aged 30 years and younger, compared to people without LFS. And while comparative risk declined with age, cancer incidence among LFS patients older than 50 years was still 10 times higher than the general population. These findings were published in December 2021 in the journal, Lancet Oncology.
Over the last decade, clinicians and researchers have developed a cancer screening program far more rigorous than that for the general population. In addition, the evidence above suggests that LFS is not a monolithic condition across the lifespan; and other evidence suggests that it is not strictly uniform among TP53-variant carriers, either. “Continued research is necessary to provide evermore personalized clinical management,” said Dr. Khincha. CGB investigators have published six papers in the past six months, revealing nuanced cancer incidence patterns for breast cancer, melanoma, soft tissue sarcoma, and other malignancies. Together, these findings highlight key distinctions within the LFS population and identify areas for improvement in TP53 variant classification and management.
Establishing the LFS Spectrum
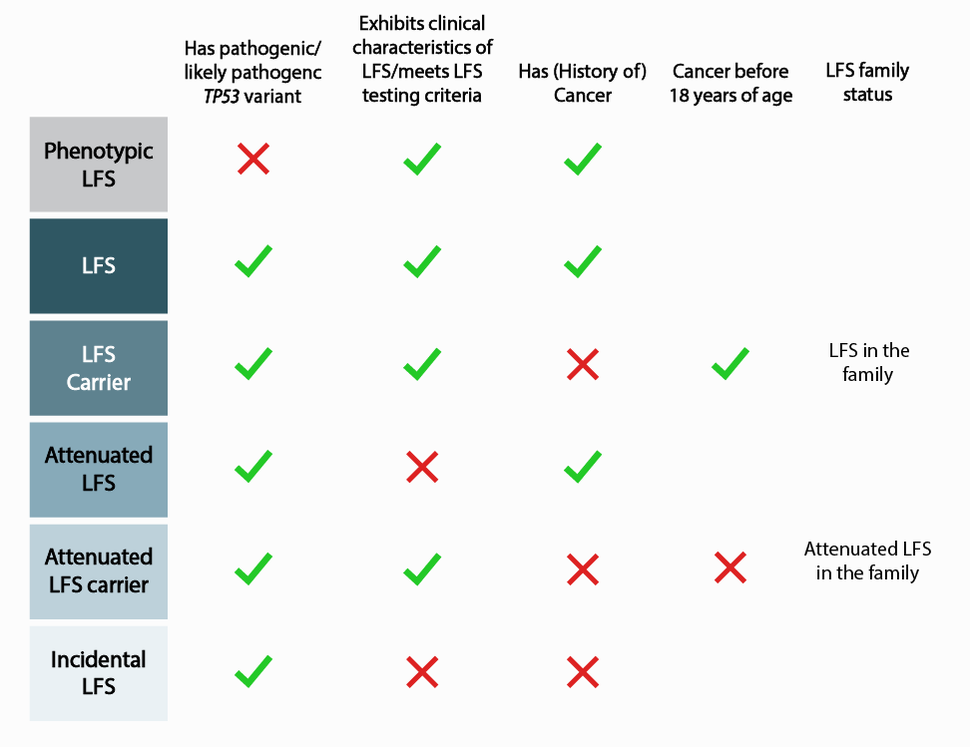
Traditionally, LFS is diagnosed using established clinical criteria, including having multiple family members with LFS-associated cancers diagnosed before the age of 45. These individuals are often recommended for genetic testing to identify the TP53 variant, if any. In addition, with evermore increasing genetic testing and smaller family sizes, clinicians are finding more individuals with TP53 variants who do not meet the clinical criteria.
Around 70 percent of families who meet classic LFS criteria carry P/LP TP53 germline variants. Clinical management for patients with known P/LP variants in TP53 includes annual whole-body MRI, and prophylactic surgery is often recommended to women to prevent breast cancer before malignancies develop or spread. “However, a patient’s particular risk profile may depend on their variant, as well as personal and family history of cancer,” said Dr. Khincha. “The current screening regimen does not address the differences in risk and management these nuances may require.”
To establish a more expansive definition of LFS, Dr. Christian Kratz of Hanover Medical School in Germany, and colleagues, analyzed data from 3034 individuals belonging to 1282 families in the TP53 database, a compilation of TP53 variant data formerly hosted by the International Agency for Research on Cancer (IARC) and transferred to the National Cancer Institute in 2021. Using these data, investigators further characterized the spectrum of phenotypic to incidental LFS, spanning from phenotypic LFS to incidental LFS (Figure 1), and published their results in December 2021 in the journal, JAMA Oncology. This nuanced characterization paves the way for improved understanding of the contributing factors that result in the observed differences and, eventually, for tailored screening protocols.
Identifying Patterns in Cancer Incidence
Drs. de Andrade and Khincha elucidated factors that may contribute to differences in cancer occurrence. For example, certain variant properties related to TP53 gene function were associated with earlier age at first and second cancer diagnosis. They also found that among patients with multiple primary cancers, second cancer diagnosis occurred more quickly in those who were older at first cancer diagnosis than in those who were younger. Finally, among some patients with two or more cancers, diagnoses occurred within a short timeframe, with no discernable pattern of occurrence. "This suggests there may be a trigger effect from either or both biological and treatment-related factors in these patients," Dr. de Andrade said.
In a related study of the same cohort, Jessica Hatton, M.S., C.G.C., a genetic counselor in CGB, and colleagues, looked at skin cancer incidence and found that melanoma was seven times more common in those with LFS, compared to the general population and several instances of cutaneous leiomyosarcoma, which is very rare in the general population. The study provides some of the first systematic evidence in support of regular skin exams for adults with LFS. Results were published in February 2022 in the Journal of Investigative Dermatology.
“Together, these studies take the first steps towards refining cancer risk modeling for personalized risk management for individuals with LFS,” said Sharon Savage, M.D., chief of CGB and co-PI of the NCI-LFS Study.
Characterizing Variants Correctly
Accurate TP53 variant classification is critical to determining appropriate medical management of cancer risk. Variants are classified as pathogenic, likely pathogenic, benign, likely benign, or a variant of uncertain significance (VUS), based on variant characteristics and evidence available through scientific literature and population databases. However, there may be clinically meaningful inconsistencies in variant classification across clinical laboratories, resulting in significant psycho-social and medical consequences. "Even though all family members who inherit LFS will carry the same variant, if they are tested at laboratories that classify the variant differently, individuals may be given significantly different clinical management recommendations,” explained Megan Frone M.S., C.G.C., genetic counselor in CGB. “And some individuals may even face difficulties receiving insurance coverage for cancer surveillance.” Additionally, inconsistent results within families may increase anxiety and confusion.
Analyzing data on more than 400 individuals from 140 families, Ms. Frone and colleagues quantified overall and clinically important inconsistencies in interpretation of germline TP53 variants, and published their results November 2021 in the journal, JCO Precision Oncology. They found 39 percent of the families had inconsistent TP53 variant interpretations; 11 percent had clinically important inconsistencies leading to potentially life-altering differences in their clinical management. This study raises awareness of the high likelihood of inconsistencies in variant interpretation and the importance of resolving them.
Further investigation is needed to identify how often and why these inconsistencies occur to improve guidelines around variant interpretation and increase consistency across clinical laboratories. The ClinGen TP53 Variant Curation Expert Panel (VCEP)—consisting of many LFS experts around the world, including Dr. Savage who serves as a co-chair and several other DCEG investigators— is one of the groups working to address these issues. ClinGen is a National Institutes of Health (NIH)-funded resource dedicated to building a central resource that defines the clinical relevance of genes and variants for use in precision medicine and research. The ClinGen TP53 VCEP curates variants using optimized classification guidelines and make their final interpretations publicly available through ClinVar, a freely accessible, public archive of reports of the relationships among human gene variations and phenotypes.
Risk Reducing Mastectomy
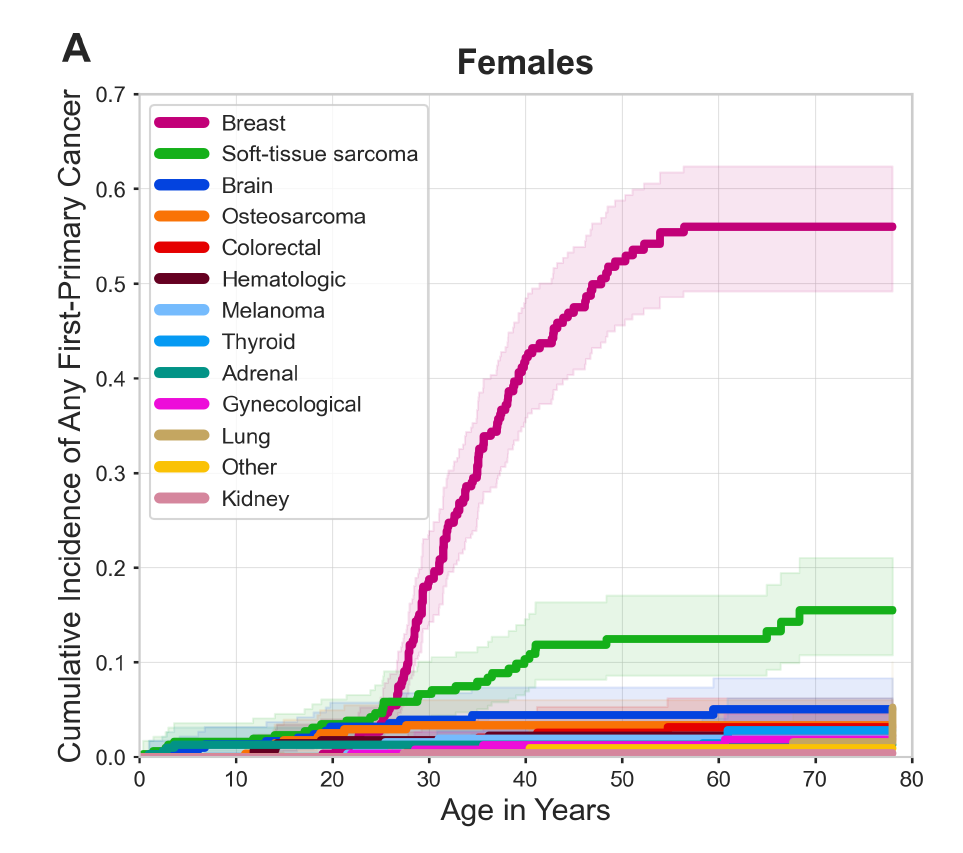
Breast cancer is the most frequently occurring malignancy in women with LFS, with a lifetime cumulative breast cancer risk of up to 56 percent by age 60 (see Figure 2) as well as elevated risk for multiple primary breast cancers. Since breast cancer is responsible for 57 percent of first cancers among women with LFS, de Andrade and Khincha examined the degree to which breast cancer drives younger age at first cancer diagnosis in this population. They found that women with LFS were half as likely to experience a first diagnosis of a non-breast cancer by age 33.7 compared to any cancer. “This suggests that if breast cancer incidence could be reduced among women with LFS, the age at first cancer diagnosis could be delayed,” Dr. Khincha said.
Risk-reducing mastectomy (RRM) is the primary means of breast cancer risk reduction for women with LFS. However, guidelines for RRM are extrapolated from data on women with pathogenic variants in BRCA1 and BRCA2.
Atara Siegel, Ph.D., former postbaccalaureate fellow in CGB and current postdoctoral fellow in the Center for Cancer Research, and colleagues conducted a study to investigate the contributing factors to RRM uptake among women with LFS and to evaluate risk in this population after undergoing the procedure. They found that women with LFS chose contralateral RRM at a higher rate than that reported for women with pathogenic variants in BRCA1 and BRCA2 (65 percent LFS v. 50 percent BRCA1/BRCA2), and bilateral RRM at comparable rates. Genetic testing prior to first breast cancer diagnosis, motherhood, and having ever breastfed were each associated with greater likelihood that a patient would choose RRM. The findings were published January 2022 in the journal, Breast Cancer Research and Treatment.
The effectiveness of RRM as a breast cancer prevention tool is promising. Among women with LFS who were diagnosed with unilateral breast cancer and did not elect RRM, there was a high rate of contralateral breast cancer: a third experienced contralateral breast cancer within 10 years; nearly half experienced contralateral breast cancer over 21 years. By contrast, none of the women who completed contralateral or bilateral RRM experienced subsequent breast cancer.
Notably, women with LFS completed bilateral RRM at a similar age as women with pathogenic variants in BRCA1 and BRCA2: median age 39 years. However, the median age of breast cancer diagnosis among women with LFS is 32 years, much younger than other breast cancer predisposition syndromes. Counseling women with LFS about RRM while they are in their teens and twenties is necessary to improve risk management in this population.
Looking to the Future
Given the centrality of TP53 variants to LFS as well as many cancers, research efforts continue to focus on improving our understanding of the gene and its contribution to cancer risk. The TP53 database plays an important role in this research. With the 2021 transfer of guardianship from IARC to NCI , under the leadership/guidance of Dr. de Andrade and others, the database was upgraded to ensure scalability, improved usability and responsive design, and enhanced navigation. A summary of the migration and future plans is in press in the journal, Cell Death and Differentiation.
As the database continues to expand and develop, “Findable, Accessible, Interoperable, Reusable” (FAIR) principles— the gold standard for data sharing—will guide data management. In addition, investigators are working to transform it into a robust, broad-ranging resource for variant curation. They will continue adding a collection of links to relevant databases, tools, publications, and other resources that may advance studies on emerging topics like the phenotypic spectrum discussed above, the role of genetic modifiers, and more.
“These efforts will improve collaboration between clinicians, researchers, and laboratories,” Dr. Savage said, “which in turn will advance our understanding of TP53 and improve clinical management of patients with LFS.”