Therapeutic Radiation Dosimetry
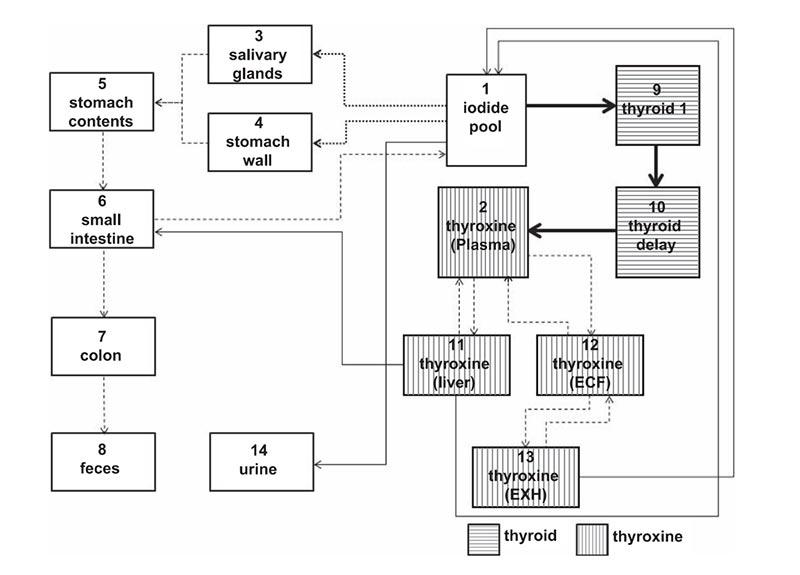
Investigators in the Radiation Epidemiology Branch (REB) develop dosimetry methods for patients undergoing therapeutic nuclear medicine procedures, in which a radiative substance (radionuclide) is introduced into the body either intravenously or orally, and that substance emits ionizing radiation within a small area inside the body. Dosimetry for nuclear medicine requires biokinetic models for given radionuclides and organ dose coefficients, which convert radionuclide uptake to absorbed dose to exposed organs and tissues.
REB investigators are developing different biokinetic models specific to epidemiological study cohorts and a library of dose coefficients by using Monte Carlo radiation transport techniques combined with computational human phantoms. By using comprehensive dose coefficients coupled with an International Commission on Radiological Protection (ICRP) reference biokinetic model, REB investigators are developing a user-friendly computer program, National Cancer Institute dosimetry system for Nuclear Medicine (NCINM). The methods have been used for dose estimates to the thyroid, active bone marrow, and other organs for a cohort of patients treated for hyperthyroidism using iodine (131I). These estimates were used .to quantify the dose-response association between 131I treatment and risk of death from specific cancers.
For more information, contact Dr. Choonsik Lee.